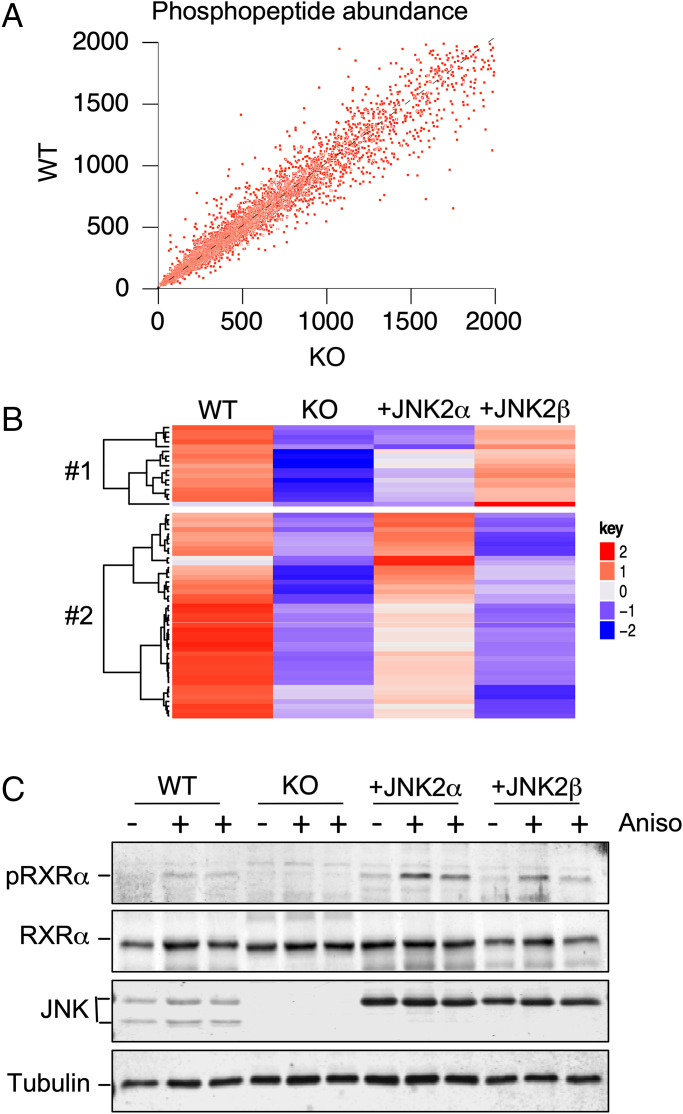
Fig. 2.
Phosphoproteomics analysis identifies JNK-dependent phosphorylation of RXRα. (A) Quantitative mass spectroscopy of multiplexed samples labeled with iTRAQ tags identified 8,955 phosphopeptides. The abundance of detected phosphopeptides in the liver of HFD-fed (16 wk) mice was examined. Data obtained from WT and hepatocyte-specific JNK1 plus JNK2 KO mice are presented. (B) Phosphorylation sites that conform to the MAPK consensus sequence (Ser-Pro and Thr-Pro) that are phosphorylated in WT liver and exhibit decreased phosphorylation in hepatocyte-specific JNK1 plus JNK2 KO liver that is partially complemented by AAV8-mediated expression of JNK2α or JNK2β are presented as a heatmap. (C) Primary hepatocytes were treated with 1 µg/mL anisomycin (Aniso, 15 min). Lysates were probed using antibodies to pSer22 RXRα (pRXRα), RXRα, JNK, and α-tubulin.