Abstract
Heat shock protein (HSP) 90, an important component of the molecular chaperone network, is closely concerned with cellular signaling pathways and stress response by participating in the process of maturation and activation of client proteins, playing a crucial role both in the normal and abnormal operation of the organism. In functionally defective tissues, programmed cell death (PCD) is one of the regulable fundamental mechanisms mediated by HSP90, including apoptosis, autophagy, necroptosis, ferroptosis, and others. Here, we show the complex relationship between HSP90 and different types of PCD in various diseases, and discuss the possibility of HSP90 as the common regulatory nodal in multiple PCD, which would provide a new perspective for the therapeutic approaches in disease.
Subject terms: Cell death, Diseases
Facts
HSP90 takes part in multiple PCD by regulating the stability and function of clients.
HSP90 inhibitors are widely used in various diseases by affecting multiple PCD.
HSP90 involves in the crosstalk of multiple PCD.
Introduction
PCD is commonly found that relates to embryonic development, immune response, aging and other physiological processes, playing an important role in cellular homeostasis by removing damaged and senescent cells [1]. Besides the well-known apoptosis, some non-apoptotic PCD forms such as ferroptosis and necroptosis are gradually discovered, which have been demonstrated that may occur together to maintain a normal cell cycle for avoiding internal and external stimuli [2–4]. Therefore, it may bring a new trend for the therapy of diseases based on the special mechanism clarification. However, the complex crosstalk of multiple PCD processes are not independent of each other but sharing a coordinated system to mediate pathology, resulting in the difficulty to affect them as expected synchronously. For example, features of necroptosis and ferroptosis can be observed in the model of acute kidney injury which could be alleviated by necroptosis inhibitor Nec-1 and ferroptosis inhibitor Fer-1, though the inhibition of ferroptosis is beneficial to necroptosis [5]. The deep studies of relevant regulatory mechanisms focusing on the crosstalk and interaction among various PCD, caspase family proteins, HSPs and other proteins that acted as common regulatory nodal have attracted widespread attention [6, 7].
Among those, HSPs, as a molecular chaperones, are synthesized to maintain homeostasis when cells are under diverse physiological states, which are considered to involve in various pathways like hormone and cell cycle [8], including constituent proteins (HSP40 and HSP90), and inducible proteins (HSP70 and HSP27) [9]. The different classes of HSPs exert specific function, and also could work together to maintaining proteostasis. As the typical representative HSPs, HSP90 and HSP70 have received the most attention belongs to the important part of the chaperone network [10]. Therefore, their roles in the pathogenesis and treatment of diseases are revealed gradually, including the regulation of proteostasis, immune and cell death pathways [11]. And the HSP90 appears to be a critical regulator of PCD.
HSP90 is the most abundant class in HSPs, whose expression could reach up to 4%-6% under stress conditions, and about 600 clients have been found in mammal [12, 13]. Generally, HSP90 fulfills the chaperone function by forming complexes with the co-chaperone and client to maintain the stability, processing and function of organisms [14]. For instance, binding to HSP90 is an indispensable part of the activation of some kinases and steroid receptors [15, 16]. As a wide range of clients, HSP90 is related to the development of lots of diseases by mediating key proteins of PCD, including receptor-interacting serine/threonine kinase (RIP) 1 in necroptosis, glutathione peroxidase (GPX) 4 in ferroptosis, Beclin-1 in apoptosis and autophagy [17–19].
The HSP90/co-chaperone/client complex is considered to be an emerging target for the treatment of diseases. So, how to block the interactions of HSP90 and client is gradually investigated, and inhibitors were proved to be the most commonly effective strategy [20–23]. Relevant genomics and experimental studies demonstrated that HSP90 inhibitors are potential antitumor agents, and some of them like geldanamycin (GA) and 17-allylamino-17-demethoxy-geldanamycin (17-AAG) have been developed and advanced into clinical trials [24, 25]. In consideration of the importance of PCD and the relation of HSP90 in the disease treatment, accumulating evidence indicates that HSP90 connects to multi-PCD such as apoptosis, necroptosis and ferroptosis [18, 26]. So, we summarized the latest researches on the correlation between HSP90 and PCD in diseases.
HSP90
HSP90 and co-chaperone
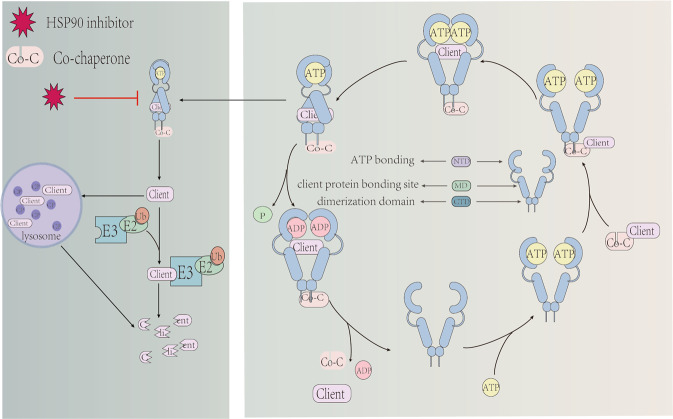
HSP90 is highly conserved during evolution, containing three conservative domains of C-terminal domain(CTD), middle domain(M-domain) and N-terminal domain(NTD) [13]. There is an ATP-binding site in NTD which is responsible for mediating the combination of co-chaperone and client based on ATPase activity [16]. The M-domain provides a site for ATP hydrolysis as well as binds to client [14], and CTD is related to the dimerization [27]. After dimerization of HSP90 connecting to ATP, the client binds to the M-domain, accompanied by NTD from open into closure [28] (Fig. 1). In general, there are four isoforms of HSP90 in cells which involves in various cellular functions, including HSP90α and HSP90β in the cytoplasm, tumor necrosis factor receptor-associated protein-1 (TRAP1) in the mitochondria and the glucose regulated protein 94 in the endoplasmic reticulum [8]. As an essential carrier of HSP90, the combination of client and HSP90 is decided by the modification of HSP90 and co-chaperone. It was reported that the moderate phosphorylation of HSP90 contributes to the maturation of most clients, but the hyperphosphorylation shows a negative effect on the chaperone machine [29]. In addition, the acetylation or the S-nitrosylation of HSP90 also blocks the interaction between HSP90 and client, which results in the inactivation and degradation of the client [12, 30].
Fig. 1. HSP90 regulates the stability and degradation of clients.
Client is regulated by forming the complex of HSP90, co-chaperone and client, whose prerequisite is the ATP-binding HSP90. When the complex complete assembly, the conformation of HSP90 changes and ATPase is activated which takes participate in the process of client. The inhibition of HSP90 affects the complex to promoting client unstable, leading the degradation of client by lysosome and ubiquitinated protease pathways.
Co-chaperone is also related to client activity, which assists with the function of HSP90 and provides selectivity for HSP90 by regulating its ATPase cycle, including p23, cell division cycle (CDC37), and so on [12, 31]. For instance, p23 is beneficial to stabilize the conformation of steroid receptors, but CDC37 is mainly responsible for kinase [32, 33]. Similarly, the inhibitors are also affecting clients selectively. The HSP90 inhibitor gedunin induces the degradation of steroid receptors in a dose-dependent manner, but has little effect on the stability of the kinase client [34]. Another inhibitor FW-04-086 regulates the cancer-related kinase to reduce proliferation and inducing apoptosis in breast cancer cells [35, 36]. Overall, the assembly of the HSP90/co-chaperone/client complex is closely relevant to various diseases, which promotes the application of HSP90 inhibitors.
HSP90 inhibitors are mainly used in cancer research, divided into NTD inhibitors, M-domain inhibitors and CTD inhibitors according to the action sites [37]. Some NTD inhibitors like GA impact the ATP binding pocket of HSP90, to promote the degradation of clients through lysosomal and ubiquitinated protease pathways [38, 39]. However, the compensatory expression of HSP70 would be induced by NTD inhibitors, which acts as a cytoprotection [40]. On the contrary, inhibitors designed for interacting with M-domain or CTD will not induce HSP70-dependent cell survival [33]. An M-domain inhibitor KA blocks the interaction between HSP90 and CDC37 by combining Cys420 with HSP90 while reserving its ATPase activity [41]. Except for the interaction with three domains of HSP90, inhibitors can also impact the co-chaperone directly. As seen in the study of Patwardhan et al., gedunin induces the cleavage of p23 by recruiting caspase7 in NTD, which decreases the interaction between HSP90 and p23 and affects the client subsequently [34, 42].
HSP90 and diseases
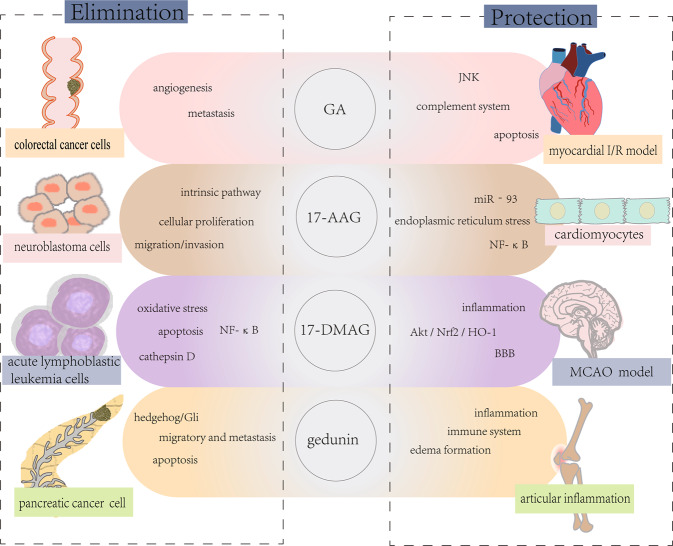
The enhancive synthesis and release of HSPs are beneficial to maintain cellular homeostasis [43, 44]. Generally, highly expressed HSP90 shows a positive cytoprotection and the cell viability is decreased by the inhibition or knockdown of HSP90, which is considered to be a feasible therapeutic target in cancer such as colorectal cancer, lung cancer and gliomas [21, 26, 45]. The main mechanism HSP90 inhibitors acted on cancer cells is mediated by inflammation and PCD, and similar events also have been found in ischemic diseases, neurodegenerative diseases, and others [7, 8, 46] (Fig. 2). For example, apoptosis could be induced in ovarian carcinoma cells when HSP90 is knockout [47]. But in the model of cerebral ischemia, treating with GA is conductive to the inhibition of apoptosis. And further more relevant information is listed in the table below [48] (Table 1).
Fig. 2. HSP90 inhibitors exert therapeutic effects in various diseases.
HSP90 inhibitors could simultaneously apply to cancer, myocardial ischemia and other diseases. On the one hand, HSP90 inhibitors protect cardiomyocytes and neurons by reducing inflammation and relevant signaling pathways. On the other hand, inhibitors eliminate cancer cells by inducing apoptosis.
Table 1.
Inhibition of HSP90 alleviated various diseases by regulating PCD.
| Inhibitors | PCD | Mechanism | Disease | Reference |
|---|---|---|---|---|
| DHQ3 | necroptosis | Activating the cascade of RIP1-RIP3-MLKL to inducing necroptosis. | breast cancer | [58] |
| FW-04-806 | apoptosis | Down-regulating HSP90 clients (HER2, Akt, RAF-1) and their phosphorylated forms (P-HER2, P-Akt); Inducing apoptosis. | breast cancer | [36] |
| C0818 | apoptosis | Degrading the clients of HSP90; Inducing caspase-dependent apoptosis. | liver cancer | [144] |
| NVPAUG922 | apoptosis | Increasing the expression of LC3II; Inducing autophagy flux. | pancreatic cancer | [145] |
| SNX-2112 | apoptosis, autophagy | Down-regulating Bcl-2 and Bcl-XL, up-regulating Bid, cleavage- caspase-9, caspase-7, caspase-3 and PARP, and activating Caspase-8; Activating mitochondria mediated and death receptor-mediated apoptosis pathways. | melanoma | [115] |
| Gedunin | apoptosis | Adding sensitivity of A549 cells to apoptosis; Inhibitng the interaction between HSP90/Beclin-1/Bcl-2, leading to the down-regulation of autophagy (Beclin-1, ATG5-12 complex, and LC3) and anti-apoptotic protein Bcl-2. | lung cancer | [19] |
| 17-AAG | apoptosis | Down-regulating c-FLIPL to promoting apoptosis. | lung cancer | [39] |
| CUDC-305 | apoptosis | Inducing the degradation of receptor tyrosine kinases and downstream signaling molecules in the PI3K/AKT and RAF/MEK/ERK pathways. | lung cancer | [25] |
| Ganetespib | apoptosis | Target autophagy by destabilizing ATG7; Mediating autophagy by destabilizing ATG7. | non-small cell lung cancer | [112] |
| FS-108 | apoptosis | Affecting the drug resistance related proteins and their downstream Akt and Erk. | non-small cell lung cancer | [60] |
| 17-AAG | apoptosis | Inhibiting the NF-κB pathway. | chronic lymphocytic leukemia | [146] |
| 17-DMAG | apoptosis | Inhibiting mTOR pathway; Promoting the transformation from LC3 I to LC3 II. | myeloma | [108] |
| shHSP90 | apoptosis | Inhibiting the AKT/GSK3β/β-catenin signaling pathway and drug resistance of cancer cells. | ovarian carcinoma | [47] |
| Radicicol | apoptosis | Activating the caspase-8 and bid-dependent pathways and mitochondria-mediated apoptosis. | ovarian carcinoma | [98] |
| Curcumin | apoptosis | Inhibiting the toxic effect of MPP on SH-SY5Y cells; Reducing apoptosis. | parkinson’s disease | [23] |
| 17-AAG | necroptosis | Decreasing the activation of RIP1/RIP3/MLKL. | heat failure | [118] |
| GA | necroptosis | Decreasing the Hsp90 expression to promoting instability of RIP1. | ischemic injury | [40] |
| HSP90i | apoptosis | Reducing infarct size; Reducing Bax/Bcl-2 ratio. | ex vivo heart perfusion system | [147] |
| GA | apoptosis | Decreasing the expression of MLK3; Down-regulating the activation of JNK. | ischemic injury | [83] |
| 17AAG | apoptosis | Inhibiting IRE-1 to promoting the expression of HAX-1. | ischemic injury | [79] |
| GA | apoptosis | Decreasing infarct size, the expression of TNF-α, and apoptosis. | ischemic injury | [80] |
| GA | apoptosis | Decreasing the expression and activation of MLK3 and FasL; | ischemic injury | [48] |
| siHSP90 | apoptosis | Up-regulating Bcl-1 and down-regulating caspase-3 and Bax. | ischemic injury | [81] |
| 17AAG | necroptosis | Blocking the interaction of RIP1 and RIP3; Reducing the phosphorylation of RIP3. | acute respiratory distress syndrome | [120] |
HSP90 inhibitors acted on the degradation and activation of clients related to PCD, then promoting the treatment of disease.
HSP90 in cancer
Compared with normal tissue, cancer cell shows a higher expression of HSP90 on mRNA and protein level [49]. It was reported that the concentration of HSP90 in plasma is associated with the malignant degree of the tumor, which is supported by the clinical data of more than 4000 patients with breast cancer [50]. Some clients may mutate or overexpress in pathological cells, which is alleviated by HSP90 inhibitors, indicating a therapeutic role in cancer. The cancer-related clients, including mitogen-activated protein kinase (MAPK) kinase, extracellular signal-regulated kinase (Erk) 1/2, and Akt [49, 51], take part in the proliferation, invasion and resistance of cancer cells [49, 52, 53]. For example, A synthetic inhibitor PF-4942847 exerts intensive effects on the viability of cancer cells by inducing apoptosis, delaying OS tumor growth and reducing lung metastasis [54]. Some recent studies shows that a higher level of HSP90 is found in plasma membrane and extracellular space of cancer cells, which participate in the invasion of tumor [52, 55]. For instance, the up-regulation of HSP90α enhances the aggressiveness of cancer cells by interacting with matrix metalloproteinase (MMP) 2 [56].
As antitumor drugs, triggering PCD is one of the most essential events, and the HSP90 inhibitors affect multiple PCD in various cancer have been verified [34]. Classical inhibitor GA has been reported that can induce autophagy and apoptosis in osteosarcoma cells by inhibiting the Akt signaling pathway [57]. Inhibitor 17-DMAG regulates the phosphorylation of Akt and Bcl-xl to increase apoptosis by targeting HSP90 whether in human or mouse lung cancer cells [49]. In addition, necroptosis is also an optional pathway for HSP90 inhibitor, which is related to the RIP1/RIP3/ mixed lineage kinase domain-like protein (MLKL) cascade [58].
Furthermore, the expressions of some resistance-related proteins like breast cancer resistance protein and survivin are regulated by HSP90 [47]. For example, multiple myeloma is a type of cancer with high recurrence and resistance, which is alleviated by blocking the interaction of HSP90 and CDC37 [59]. Due to the compatibility of unstable kinases with HSP90, inhibitors are beneficial to eliminate resistance after kinase inhibition in the treatment of cancer [60, 61]. 17-AAG affects radiosensitivity which is a pivotal point of clinical treatment by increasing the F-box protein 6 mediated polyubiquitination of CD147 [59]. HSP90 also is a key mediator of IFN-γ-induced adaptive immune resistance by regulating the expression of immune checkpoints like programmed death ligand 1, which exerts physiologic and pathologic effects in autoimmunity and immune escape [53]. In addition, some classical signaling pathways in cancer are affected by HSP90, such as Akt and NF-κB pathway [51, 62, 63]. In general, HSP90 inhibitors have been already demonstrated that against several cancers effectively due to its regulatory of key proteins in the development of cancer.
HSP90 in neurodegenerative diseases
Protein homeostasis is one of the important factors of neuronal state, and its disorder may be the main reason of some conformational diseases. It was reported that the misfolding and aggregation of symbolic proteins are partly related to HSP90 chaperone machines [64]. The characteristic Tau tangles and β-amyloid deposition are co-locating with HSP90 in Alzheimer’s disease patients, whose aggregation and degradation are regulated by HSP90 [65]. According to the study of Chen et al., the neurotoxicity caused by β-amyloid could be reduced by the HSP90 inhibitor, further promoting the normalization of synaptic function [66]. In addition, the complex consisting of HSP90, p23 and PHD2 shows a significant increase both in vitro and vivo PD models, and the clinical symptomatic relief of PD can be obtained when the assembly of complex is inhibited [67]. And the HSP90/FK506-binding protein (FKBP) 51 machine is demonstrated that involves in the activity of GR, which is regarded as a regulatory of psychiatric diseases like depression [68, 69]. In general, HSP90 chaperone machine is widely participating in the development and treatment of a variety of neurodegenerative diseases by regulating the balance between HSP90 and different co-chaperones.
Tau participates in the assembly and stabilization of microtubules and performed hyperphosphorylation and aggregation in neurodegenerative diseases [70]. HSP90 plays an essential role in the process of folding, degradation and aggregation of tau, and the relevant co-chaperones include FKBP51 and CDC37 [35, 65]. Oligomerization of Tau is synergistically triggered by the HSP90/FKBP51 machine, which is conductive to the accumulation of the toxic Tau [71]. The co-chaperone Aha1 also increases the aggregation and toxicity of Tau [72]. In contrast, protein phosphatase 5 and cyclophilin 40 promote phosphorylation and decomposition of the aggregating Tau [65].
Similarly, TAR DNA binding protein (TDP) 43 is also a client of HSP90, whose aberrant aggregation is a signature of amyotrophic lateral sclerosis [73]. As the study of Lin et al., the toxicity of TDP-43 is regulated by the specific interaction between HSP90/stress-inducible phosphoprotein (Sti) 1 machine and TDP-43 [74, 75]. The appropriate expression of Sti1 reduces the toxicity of TDP-43, while the abnormal Sti1 promotes the dysfunction of the neuron. In addition, HSP90/CDC37 is associated with the nuclear location of TDP-43 [76]. The all above suggest that the HSP90 machine plays a significant effect on neurodegenerative diseases.
HSP90 in cerebro-cardiovascular diseases
The function of endothelial nitric oxide synthase, endothelial growth factor receptor and other vascular-related proteins are regulated by HSP90, which is related to the circulatory system [77]. It was reported that the Erk and Heme-Oxygenase-1 signaling are activated by suppressing the interaction of CDC37/HSP90, which could effectively reduce the infarct area, fibrosis and macrophage infiltration in the myocardial ischemia/perfusion model [78]. And inhibiting apoptosis is also the mechanism by which HSP90 inhibitors exert their protective effect [79]. GA reduces apoptosis in the process of myocardial injury by mediating the complement system and JNK signaling pathway [80].
HSP90 is also involved in cerebrovascular disease. Numerous studies demonstrated that HSP90 is significantly increasing in the model of cerebral ischemia-reperfusion injury [81]. The HSP90 inhibitor GA shows obvious neuronal protection both in the whole and focal cerebral I/R model, owing to the up-regulation of HSP70 and HSP25 in neurons [82]. In the model of four-vessel occlusion ischemic on rat, the intensive association of HSP90 and MLK3 is reversed by GA, which exerts a strong neuroprotection [83]. In addition to necroptosis, the inhibition of HSP90 is also related to apoptosis, autophagy and other PCD in stroke [48]. The another therapeutic mechanism of HSP90 inhibitor for cerebrovascular disease is maintaining the function of blood-brain barrier (BBB), which easily affects by inflammation under hypoxia condition [84]. In the study of Zhang et al., injecting siHSP90 alleviates oxidative stress and inflammation in the model of I/R[81]. And 17-DMAG is considered to play a protective role by down-regulating MMP9 to maintain BBB [85].
HSP90 in other diseases
Some studies have been demonstrated that HSP90 is involved in pulmonary fibrosis, and 17-AAG could decrease fibrosis and MMP activity to alleviate idiopathic pulmonary fibrosis [86]. In addition, HSP90 is associated with the activation of glial cells in the spinal cord that are involved in the typical pain signaling cascade events [87]. Treating with 17-DMAG could relieve abnormal pain induced by exercise and monoarthritis, which is related to inflammatory cascade [88, 89]. In the case of low oxygen, HSP90α combines with the low-density lipoprotein receptor-related protein 1 cytoplasm tail to stabilize the receptor on the cell surface, and the Hsp90β is secreted into the cell space to increase cell movement, to promote wound healing by interacting with the low-density lipoprotein-related protein-1 receptor signaling [90].
Overall, the treatment strategy of targeting HSP90 has been widely studied, but its specific role in diseases remains not fully clarified. HSP90 is generally thought to be up-regulated in response to stress rather than cause disease. However, the up-regulation of HSP90 is beneficial to the survival of cancer cells and the mutation of cancer-related proteins, which is the reason for the use of inhibitors in cancer treatment. Furthermore, HSP90 in cancer cells is distinguished from the ordinary cells by the greater activities, extracellular localization and special post-translational modifications [45]. There are more than 30 different post-translational modifications of HSP90 in cells, and the change of modification induced by exogenous stimulus might be one of the mechanisms of its negative role in diseases. For example, alcohol induces the acetylation of HSP90 to decrease the interaction with eNOS, further leading to liver injury [91]. And the S-nitrification of HSP90 is observed in atherosis [30]. And for neurodegenerative diseases like AD, the imbalance of the chaperone system including HSP90 might be an important factor in its pathogenesis. In general, HSP90 has a unique mechanism under the disease state, but its specific mechanism still needs to be supplemented.
The relationship of HSP90 and multiple PCD
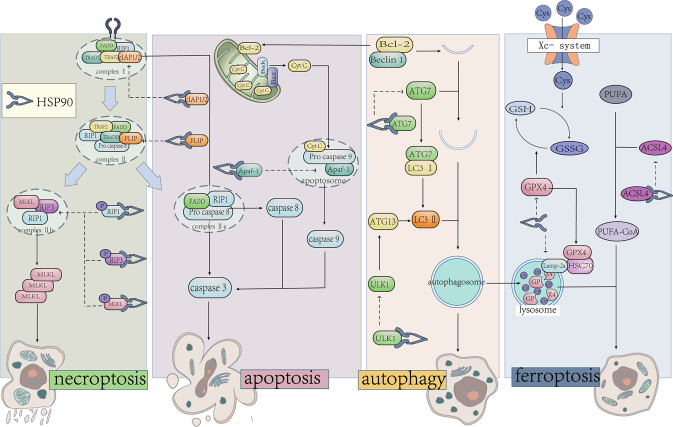
As a fundamental process of cells, there are complex connections among multiple PCD, and HSP90 is one of the common regulatory nodal in apoptosis, autophagy, necroptosis, ferroptosis and other PCD. (Fig. 3).
Fig. 3. HSP90 mediates multiple PCD.
HSP90 plays an important role in the PCD regulation network. Forming complex with co-chaperone and clients to regulate their phosphorylation and stability is the main mechanism. And the clients of HSP90 are distributed in various processes, including the RIP1, RIP3 and MLKL in necroptosis, Bcl-2 and cellular-FLICE inhibitory protein (FLIP) in apoptosis, ULK1 and ATG7 in autophagy, GPX4 and ACLS4 in necroptosis.
HSP90 and apoptosis
Apoptosis is the earliest cognitive PCD, which occurs ubiquitously in various diseases by eliminating aberrant cells through intrinsic and extrinsic pathways [92]. The intrinsic pathway is caused by internal stress signals like DNA damage, resulting in mitochondrial outer membrane permeabilization(MOMP) [93]. Then, the Cyt C released from mitochondria assembles the apoptotic bodies to facilitate the activation of the caspase cascade and further induce apoptosis ultimately [94]. And the extrinsic pathway can promote the assembly of the DISC to induce the maturation of caspase8 and triggers the caspase cascade to mediate apoptosis [95]. With further study of the mechanism, both the intrinsic and extrinsic pathways are believed to be regulated by HSP90.
HSP90 is closely related to multiple processes of intrinsic apoptosis. For example, HSP90 decreases the release of Cyt C by interacting with Bcl-2 [96]. And the G-TPP could cause MOMP and the release of Cyt C by inhibiting TRAP-1 [97]. HSP90 also takes part in the assembly of apoptotic bodies by regulating apoptotic protease activating factor 1 and is associated with the cleavage and function of numerous caspases, including caspase3, 6, 9, and so on [98–101]. Some N-terminal inhibitors also could regulate apoptosis by activating HSF-1, which is beneficial for the up-regulation of HSP70 and HSP27 [102].
In the extrinsic pathway, c-FLIP is an essential negative regulatory which blocks the activation of caspase 8/10 in DISC [103]. 17-AAG induces apoptosis in lung cancer cells by decreasing the expression of c-FLIP, suggesting that HSP90 may mediate apoptosis by impacting the degradation of c-FLIP [39, 104, 105]. In addition, the MG132, a proteasome inhibitor, inhibits the down-regulation of c-FLIP in CALu-1 cells after 17-AAG treatment [39], which proves the regulation of 17-AAG in apoptosis owes to mediating the degradation of c-FLIP via the proteasome pathway.
HSP90 and autophagy
Autophagy is defined as a process of removing damaged proteins and organelles by engulfing them into vesicles to form autophagosomes [106], including microautophagy, chaperone-mediated autophagy (CMA), and macroautophagy. CMA is described as a process that degrades specific clients by transporting them into lysosomes when recognized by lysosome-associated membrane protein type (LAMP) 2a [107]. And the stability of LAMP-2a is regulated by HSP90 [108]. Generally, autophagy is closely related to the degradation of clients which includes lysosome and ubiquitinated protease pathways. For example, IKK is selectively degraded when HSP90 is inhibited, and the inhibition of ATG5 can reverse IKK degradation [38], suggesting that HSP90 may lead to the degradation of IKK through the lysosomal pathway. In contrast, the protease inhibitor MG132 interrupts the degradation of RIP3, which is related to the ubiquitinated protease pathway [41].
In addition, HSP90 is associated with several key proteins in macroautophagy, such as ULK1, Beclin-1, ATG7, and so on [109]. ULK1, a mammalian homolog of ATG1, is involved in the nucleation and extension process of the autophagosome, whose function is maintained by forming a complex with HSP90 and CDC37 on its N-terminal kinase domain [110]. On one hand, the interaction between HSP90 and ULK1 contributes to the autophosphorylation of ULK1 at Ser1047, which is interrupted by the treatment of HSP90 inhibitor [110]. On the other hand, 17-AAG does not affect the mRNA level of ULK1, but it could decrease the homeostasis of ULK1, indicating that the interaction between HSP90 and ULK1 is in favor of the stabilization of ULK1. In addition, ATG13 is a substrate of ULK1 and could be phosphorylated by ULK1 at Ser318, whose phosphorylation needs HSP90/CDC37/ULK1 complex [110, 111]. HSP90 is also related to the localization of ATG13, which is described as translocating to damaged mitochondria to mediate its elimination [112].
Beclin-1 is a key regulatory in early autophagy whose function depends on HSP90 [113]. HSP90 forms a complex with Beclin-1 through an evolutionarily conserved domain to maintaining its stability and phosphorylation [69, 114]. GA separates Beclin-1 from HSP90, further promoting its degradation through the ubiquitinated protease pathway [114]. In addition, SNX-2112 could inhibit the formation of the ATG7/caspase9 complex, which is a key to the alteration of apoptosis and autophagy [115]. GA also affects the interaction between HSP90 and ATG7 by disrupting the stability of ATG7 [112]. It was also reported that the regulation of HSP90 inhibitor on autophagy is related to the rate of LC3II/I and the formation of autophagosomes [116, 117].
HSP90 and necroptosis
Compared to necrosis that had been considered as unregulated, increasing evidence indicates that there is a caspase-independent PCD defined as necroptosis, characterized by the loss of cell membrane integrity and release of cytoplasmic contents [3]. Necroptosis is strictly regulated by the RIP1/RIP3/MLKL pathway, and the HSP90 inhibitor involves in the stability, phosphorylation, and expression levels of RIP1, RIP3 and MLKL in necroptosis [17, 40]. For example, the higher expression and hyperphosphorylation of RIP1, RIP3 and MLKL in the model of heart failure would be reversed by HSP90 inhibitor [118]. The expression of RIP1 and RIP3 is inhibited by 17-AAG which could be reversed by CDC37 knockdown, suggesting that activation of RIP3 is related to the HSP90/CDC37 complex [119]. In addition to CDC37, p23 is also co-located with RIP3, affecting its phosphorylation [120]. The correlation between HSP90 and RIP3 expression remains controversial. Although most studies have shown that HSP90 inhibitors simultaneously reduce the phosphorylation of RIP3 in disease models [40], 17-AAG has no effect on the abundance of RIP3 and merely regulates its function [119]. The duration of HSP90 inhibitors effecting on cells is also critical to the function of RIP3, which demonstrated that short-term inhibition of HSP90 may lead to conformational changes of RIP3, and the degradation of RIP3 via ubiquitinated protease pathway is mediated by long-term inhibition of HSP90 [41, 121, 122].
Activity of HSP90 is also essential for the processes of phosphorylation, oligomerization and membrane translocation of MLKL [123, 124]. Previous studies show that MLKL is phosphorylated at Ser227 and Ser358 during necroptosis, which is strongly inhibited by 17-AAG [122]. HSP90 is also vital to promote the oligomerization and translocation of MLKL, though the interaction with HSP90 is weak or transitory [122–124]. Considering that MLKL is downstream of this cascade, the RIP3-deficient fibroblast cell is used for determining that HSP90 inhibitors can directly regulate MLKL to mediate necroptosis [122]. In general, HSP90 takes part in multiple processes of necroptosis regulated by different HSP90 inhibitors.
HSP90 and ferroptosis
Ferroptosis is an emerging iron-dependent cell death way, which is characterized by membrane rupture and vesiculation, mitochondrial atrophy, decrease of the mitochondrial ridge, and an increase in membrane density [2]. Some studies show that HSP90 is a potential target for ferroptosis, while its role in ferroptosis remains controversial [18, 125]. Su et al. found that HSP90 inhibitor GA promotes the depletion of GSH to accelerates the occurrence of ferroptosis [125]. On the contrary, another HSP90 inhibitor CDDO is beneficial in reducing ferroptosis [18]. According to the study of Wu et al., CDDO significantly inhibits ferroptosis by affecting the expression of GPX4, which is one of the most classical biomarkers of ferroptosis [18, 126].
A previous study shows that GPX4 is the substrate of CMA, which is affected by LAMP-2a [108, 127]. The overexpressed LAMP-2a promotes CMA to decrease the expression of GPX4, which enhances the sensitivity to erastin-induced ferroptosis, and an obvious high expression of GPX4 is detected in LAMP-2a knockdown cells [128]. The interaction of HSP90 and Lamp-2a shows a significant increasing when the cells treat with erastin [18, 108]. Another latest study also demonstrates that HSP90 plays a positive role in ferroptosis by regulating ACSL4. As a key biomarker of ferroptosis, ACSL4 involves in lipid peroxidation, whose expression is related to the interaction of HSP90 and Drp1 [129].
HSP90 and other PCD (PANoptosis)
In addition to the above, some studies have shown that HSP90 is also associated with other PCD, such as pyroptosis. Pyroptosis is a recently discovered PCD accompanied by an inflammatory response, which is characterized by rapid plasma membrane rupture, DNA damage, and the release of pro-inflammatory cytokine [130]. And the NLRP3/caspase-1/GSDMD pathway is considered to be the key to regulating pyroptosis. Inhibition of NLRP3 inflammasome is a reliable therapeutic target for a variety of inflammatory diseases, and the interaction of HSP90 and NLRP3 is related to its stability and activation, further regulates downstream IL-1β secretion and pyroptosis[131]. Normally, NLRP3 is inactivated when it binds to HSP90, and upon receiving an inflammatory signal, the interaction is blocking to prompting the activation of NLRP3 and the initiation of subsequent inflammatory cascades [132]. Finally, the complex correlations between HSP90 and emerging PCD, like Cuproptosis, NETosis and PANoptosis, remain to be further investigated.
Crosstalk between HSP90 and multiple PCD
In the studies of targeting for HSP90, some researchers have found that HSP90 may involve in the selection of multiple PCD. As reported, the inhibition of HSP90 could transform necroptosis induced by DD receptor into apoptosis [133]. And different HSP90 inhibitors selectively activate or inhibit multiple PCD, even in the same objects. For example, DHQ3 induces necroptosis by activating the RIP1/RIP3/MLKL pathway in human breast cancer cells, while 17-DR induces caspase-3 and caspase-8-dependent apoptosis [58]. In addition, HSP90 inhibitors also appear under different regulations when affecting multiple PCD simultaneously (Table 2). According to the research of Yan et al., HSP90 is a critical regulator of necroptosis and apoptosis, whose inhibitor alleviates necroptosis and promote the activation of apoptosis [97]. In another study, the inhibition of HSP90 could reduce both necroptosis and apoptosis in nucleus pulposus-derived stem cells [17]. The specific regulation is both related to types of inhibitors and pathological context of cell. For, example, 17-AAG, a widely used inhibitor, induces apoptosis in lung cancer cells but reduces apoptosis in a rat CCI model [39].
Table 2.
HSP90 inhibitors regulated multiple PCD simultaneously.
| HSP90 inhibitors | PCD | Function | Cells | Reference |
|---|---|---|---|---|
| gedunin | autophagy | reduce | Lung cancer cells | [19] |
| apoptosis | induce | |||
| GA | autophagy | induce | Osteosarcoma cells | [57] |
| apoptosis | induce | |||
| GA | autophagy | induce | kidney cells | [148] |
| apoptosis | reduce | |||
| SNX-2112 | autophagy | induce | Melanoma cells | [115] |
| apoptosis | induce | |||
| 17-AAG | autophagy | induce | Hepatocellular carcinoma | [149] |
| apoptosis | induce | |||
| 17-DMAG | autophagy | reduce | B-ALL cells | [150] |
| apoptosis | induce | |||
| G-TPP | autophagy | induce | Hep3B cells | [97] |
| apoptosis | induce | |||
| necroptosis | induce | |||
| BIIB021 | apoptosis | reduce | NPSCs | [17] |
| necroptosis | reduce | |||
| CDDO | necroptosis | reduce | HT-22 | [18] |
| ferroptosis | reduce |
The inhibitors regulated different PCD in the same objects. And the different inhibitors mediated the same PCD in a similar or contrast way.
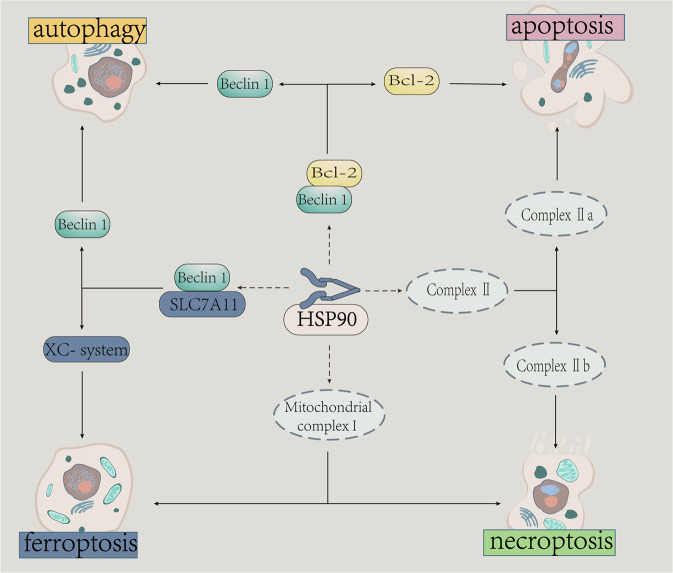
With the deepening of relevant researches, parts of complexes and proteins are regarded as key nodal in the complex regulatory network of PCD, selectively promoting cells towards different PCD, including complex II, Beclin1-Bcl 2, and some caspases [134].(Fig. 4). Mechanistically, necroptosis and extrinsic apoptosis share a common initiation, which is separated by the assemble of complex I and II. cIAP1/2 is one of the key regulatory, exerting anti-apoptotic function [135]. When cIAP1/2 is inhibited, the assembly of complex IIa is increasing to activate the caspase cascade and induce apoptosis, which is consisted of RIP1, FADD, and caspase-8 [136]. When RIP1 deubiquitinated and caspase-8 inhibited, the assembly of complex IIb induces necroptosis promoted after the binding of RIP3 and RIP1. Inhibitor CDDO could inhibit necroptosis by disrupting the formation of complex IIb [18]. Another determining element is the balance of c-FLIP and RIP1/RIP3/MLKL whose expression and function are regulated by HSP90 [39].
Fig. 4. HSP90 relates to the selection of cell death forms in cells.
There are complex connects among multiple PCD, and the relation of them are not fixed but flexible which depends on the physiological and pathological conditions. HSP90 regulates some key procedures of the PCD transformation, including the Beclin1/Bcl-2 complex, Beclin1/SLC7A11 complex, mitochondrial complex I and complex II.
Beclin-1/Bcl-2 is also an important common regulatory nodal between apoptosis and autophagy, whose stability requires the involvement of HSP90 [137]. Gedunin targets HSP90 to mediate the interaction of Beclin-1/Bcl-2 and endoplasmic reticulum stress, then regulates the transformation between apoptosis and autophagy [19]. Beclin1 could also disrupt the Xc- system by interacting with SLC7A11, which may mediate the correlation between autophagy and ferroptosis [138]. In addition, mitochondrial complex I is an important nodal for autophagy, necroptosis and ferroptosis, which could be inhibited by celastrol [139, 140]. In addition to apoptotic activity, some caspases can also participate in other physiological activities [141]. For example, caspase 9 is involved in the initial autophagosome formation when its apoptotic activity is inhibited by interacting with ATG7, which could be regulated by an HSP90 inhibitor [6, 112, 142].
Conclusions and perspectives
Since PCD is closely related to the process of disease development, drugs usually take effect by eliminating aberrant cells and protecting normal cells. The HSP90 inhibitors have been used in the research of diseases due to the excellent effect in this respect. However, there are still many problems need to be solved in this process. For example, some N-terminal inhibitors regulate PCD by binding to the NTD of HSP90, which would up-regulate the HSP70-related cytoprotection to cause unsatisfactory results [33]. This phenomenon promotes the development of CTD and MD inhibitors which would not induce this protection. Furthermore, although HSP90 inhibitors have shown promising therapeutic effects in related mechanistic studies, they have not performed as expected in clinical trials. The dissatisfactory specificity of inhibitor is one of the main limiting factors, which is caused by the sequence identity of the four isoforms, especially the 85% similarity between HSP90α and HSP90β [8]. So, the researches on isoform-selective inhibitors are still continually deepened, which is conductive to reducing the pan-inhibition of Hsp90. In the study of Chaudhury et al., the reported complex shows the greatest selectivity towards HSP90β, and even more than 370 fold compared with HSP90α [143]. In general, although the problems of targeting HSP90 in disease treatment have revealed, it still be a feasible approach because these problems could be gradually solved.
Overall, according to relevant studies of targeting HSP90 for diseases therapy, the feasibility and prospects are as follows: [1] Due to the complex interactions of HSP90, co-chaperone and clients, HSP90 inhibitors are designed to regulating key proteins in diseases by blocking the combination. However, the current studies mainly focus on single PCD, and the effect of inhibitors on various PCD is complex. How to select the appropriate inhibitors to promote cell survival or inhibit cell activity as a whole may be one of the important problems for the situation of HSP90 as a therapeutic target. [2] As far as cancer treatment is concerned, the mechanism of targeting HSP90 to regulate apoptosis and necroptosis has already presented lots of relevant studies, but the relationship between HSP90 and some emerging cell death pathways should be further explored, including ferroptosis, pyroptosis, and cuproptosis. [3] N-terminal inhibitors of HSP90 can activate the heat shock response and increase the expression of HSP70, which exerts strong cellular protection. The low-dose inhibitors may acutely activate the heat shock response to alleviate disease without extensive cytotoxicity, so HSP90 inhibitor with an appropriate dose may be a vital event in disease treatment. In general, the role of HSP90 in disease and regulation in multiple PCD still has great prospects.
Author contributions
Concept and design: LF and PCW. Writing, review and revision of the manuscript: PCW, ZFY, LHL and LF. Suggestions: LL and YYT. All authors have agreed on the final version to be published. All authors reviewed and approved the manuscript.
Funding
National Natural Science Foundation of China (81704064); Outstanding Youth Program of Scientific Research of Hunan Provincial Department of Education (20B434); Natural Science Foundation of Hunan Province (2022JJ30436); Natural Science Foundation of Changsha City (kq2202259); 2021 Hunan University of Traditional Chinese Medicine Graduate Innovation Project (2021CX53); 2022 Hunan University of Traditional Chinese Medicine Graduate Innovation Project (2022CX91).
Data availability
Original data used for this report (although not applicable) will be made available upon request to the corresponding author.
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Edited by Boris Zhivotovsky
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bai Y, Lam HC, Lei X. Dissecting Programmed Cell Death with Small Molecules. Acc Chem Res. 2020;53:1034–45. doi: 10.1021/acs.accounts.9b00600. [DOI] [PubMed] [Google Scholar]
- 2.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–72. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol. 2010;11:700–14. doi: 10.1038/nrm2970. [DOI] [PubMed] [Google Scholar]
- 4.Li M, Wang ZW, Fang LJ, Cheng SQ, Wang X, Liu NF. Programmed cell death in atherosclerosis and vascular calcification. Cell Death Dis. 2022;13:467. doi: 10.1038/s41419-022-04923-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muller T, Dewitz C, Schmitz J, Schroder AS, Brasen JH, Stockwell BR, et al. Necroptosis and ferroptosis are alternative cell death pathways that operate in acute kidney failure. Cell Mol Life Sci. 2017;74:3631–45. doi: 10.1007/s00018-017-2547-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han J, Hou W, Goldstein LA, Stolz DB, Watkins SC, Rabinowich H. A Complex between Atg7 and Caspase-9: A NOVEL MECHANISM OF CROSS-REGULATION BETWEEN AUTOPHAGY AND APOPTOSIS. J Biol Chem. 2014;289:6485–97. doi: 10.1074/jbc.M113.536854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou Y, Liao J, Mei Z, Liu X, Ge J. Insight into Crosstalk between Ferroptosis and Necroptosis: Novel Therapeutics in Ischemic Stroke. Oxid Med Cell Longev. 2021;2021:9991001. doi: 10.1155/2021/9991001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoter A, El-Sabban ME, Naim HY The HSP90 Family: Structure, Regulation, Function, and Implications in Health and Disease. Int J Mol Sci. 2018;19. [DOI] [PMC free article] [PubMed]
- 9.Hall JA, Forsberg LK, Blagg BS. Alternative approaches to Hsp90 modulation for the treatment of cancer. Future Med Chem. 2014;6:1587–605. doi: 10.4155/fmc.14.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Genest O, Wickner S, Doyle SM. Hsp90 and Hsp70 chaperones: Collaborators in protein remodeling. J Biol Chem. 2019;294:2109–20. doi: 10.1074/jbc.REV118.002806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vostakolaei MA, Abdolalizadeh J, Hejazi MS, Kordi S, Molavi O. Hsp70 in Cancer: Partner or Traitor to Immune System. Iran J Allergy Asthma Immunol. 2019;18:589–604. doi: 10.18502/ijaai.v18i6.2172. [DOI] [PubMed] [Google Scholar]
- 12.Prodromou C. Mechanisms of Hsp90 regulation. Biochem J. 2016;473:2439–52. doi: 10.1042/BCJ20160005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel HJ, Modi S, Chiosis G, Taldone T. Advances in the discovery and development of heat-shock protein 90 inhibitors for cancer treatment. Expert Opin Drug Disco. 2011;6:559–87. doi: 10.1517/17460441.2011.563296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee K, Thwin AC, Nadel CM, Tse E, Gates SN, Gestwicki JE, et al. The structure of an Hsp90-immunophilin complex reveals cochaperone recognition of the client maturation state. Mol Cell. 2021;81:3496–508.e5. doi: 10.1016/j.molcel.2021.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu XA, Wang X, Zhuo W, Jia L, Jiang Y, Fu Y, et al. The regulatory mechanism of a client kinase controlling its own release from Hsp90 chaperone machinery through phosphorylation. Biochem J. 2014;457:171–83. doi: 10.1042/BJ20130963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taipale M, Krykbaeva I, Koeva M, Kayatekin C, Westover KD, Karras GI, et al. Quantitative analysis of HSP90-client interactions reveals principles of substrate recognition. Cell. 2012;150:987–1001. doi: 10.1016/j.cell.2012.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu B, Zhang S, Liu W, Wang P, Chen S, Lv X, et al. Inhibiting Heat Shock Protein 90 Protects Nucleus Pulposus-Derived Stem/Progenitor Cells From Compression-Induced Necroptosis and Apoptosis. Front Cell Dev Biol. 2020;8:685. doi: 10.3389/fcell.2020.00685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu Z, Geng Y, Lu X, Shi Y, Wu G, Zhang M, et al. Chaperone-mediated autophagy is involved in the execution of ferroptosis. Proc Natl Acad Sci USA. 2019;116:2996–3005. doi: 10.1073/pnas.1819728116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hasan A, Haque E, Hameed R, Maier PN, Irfan S, Kamil M, et al. Hsp90 inhibitor gedunin causes apoptosis in A549 lung cancer cells by disrupting Hsp90:Beclin-1:Bcl-2 interaction and downregulating autophagy. Life Sci. 2020;256:118000. doi: 10.1016/j.lfs.2020.118000. [DOI] [PubMed] [Google Scholar]
- 20.Mielczarek-Lewandowska A, Hartman ML, Czyz M. Inhibitors of HSP90 in melanoma. Apoptosis. 2020;25:12–28. doi: 10.1007/s10495-019-01577-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gallerne C, Prola A, Lemaire C. Hsp90 inhibition by PU-H71 induces apoptosis through endoplasmic reticulum stress and mitochondrial pathway in cancer cells and overcomes the resistance conferred by Bcl-2. Biochim Biophys Acta. 2013;1833:1356–66. doi: 10.1016/j.bbamcr.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 22.Normant E, Paez G, West KA, Lim AR, Slocum KL, Tunkey C, et al. The Hsp90 inhibitor IPI-504 rapidly lowers EML4-ALK levels and induces tumor regression in ALK-driven NSCLC models. Oncogene. 2011;30:2581–6. doi: 10.1038/onc.2010.625. [DOI] [PubMed] [Google Scholar]
- 23.Sang Q, Liu X, Wang L, Qi L, Sun W, Wang W, et al. Curcumin Protects an SH-SY5Y Cell Model of Parkinson’s Disease Against Toxic Injury by Regulating HSP90. Cell Physiol Biochem. 2018;51:681–91. doi: 10.1159/000495326. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Z, Jing J, Ye Y, Chen Z, Jing Y, Li S, et al. Characterization of the dual functional effects of heat shock proteins (HSPs) in cancer hallmarks to aid development of HSP inhibitors. Genome Med. 2020;12:101. doi: 10.1186/s13073-020-00795-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bao R, Lai CJ, Wang DG, Qu H, Yin L, Zifcak B, et al. Targeting heat shock protein 90 with CUDC-305 overcomes erlotinib resistance in non-small cell lung cancer. Mol Cancer Ther. 2009;8:3296–306. doi: 10.1158/1535-7163.MCT-09-0538. [DOI] [PubMed] [Google Scholar]
- 26.Gaponova AV, Nikonova AS, Deneka A, Kopp MC, Kudinov AE, Skobeleva N, et al. A Novel HSP90 Inhibitor-Drug Conjugate to SN38 Is Highly Effective in Small Cell Lung Cancer. Clin Cancer Res. 2016;22:5120–9. doi: 10.1158/1078-0432.CCR-15-3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scheufler C, Brinker A, Bourenkov G, Pegoraro S, Moroder L, Bartunik H, et al. Structure of TPR Domain–Peptide Complexes. Cell. 2000;101:199–210. doi: 10.1016/S0092-8674(00)80830-2. [DOI] [PubMed] [Google Scholar]
- 28.Biebl MM, Buchner J. Structure, Function, and Regulation of the Hsp90 Machinery. Cold Spring Harb Perspect Biol. 2019;11:a034017. [DOI] [PMC free article] [PubMed]
- 29.Mollapour M, Neckers L. Post-translational modifications of Hsp90 and their contributions to chaperone regulation. Biochim Biophys Acta. 2012;1823:648–55. doi: 10.1016/j.bbamcr.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao S, Tang X, Miao Z, Chen Y, Cao J, Song T, et al. Hsp90 S-nitrosylation at Cys521, as a conformational switch, modulates cycling of Hsp90-AHA1-CDC37 chaperone machine to aggravate atherosclerosis. Redox Biol. 2022;52:102290. doi: 10.1016/j.redox.2022.102290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dahiya V, Agam G, Lawatscheck J, Rutz DA, Lamb DC, Buchner J. Coordinated Conformational Processing of the Tumor Suppressor Protein p53 by the Hsp70 and Hsp90 Chaperone Machineries. Mol Cell. 2019;74:816–30.e7. doi: 10.1016/j.molcel.2019.03.026. [DOI] [PubMed] [Google Scholar]
- 32.Obermann WMJ. A motif in HSP90 and P23 that links molecular chaperones to efficient estrogen receptor alpha methylation by the lysine methyltransferase SMYD2. J Biol Chem. 2018;293:16479–87. doi: 10.1074/jbc.RA118.003578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Terracciano S, Russo A, Chini MG, Vaccaro MC, Potenza M, Vassallo A, et al. Discovery of new molecular entities able to strongly interfere with Hsp90 C-terminal domain. Sci Rep. 2018;8:1709. doi: 10.1038/s41598-017-14902-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patwardhan CA, Fauq A, Peterson LB, Miller C, Blagg BS, Chadli A. Gedunin inactivates the co-chaperone p23 protein causing cancer cell death by apoptosis. J Biol Chem. 2013;288:7313–25. doi: 10.1074/jbc.M112.427328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gracia L, Lora G, Blair LJ, Jinwal UK. Therapeutic Potential of the Hsp90/Cdc37 Interaction in Neurodegenerative Diseases. Front Neurosci. 2019;13:1263. doi: 10.3389/fnins.2019.01263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang W, Ye M, Zhang LR, Wu QD, Zhang M, Xu JH, et al. FW-04-806 inhibits proliferation and induces apoptosis in human breast cancer cells by binding to N-terminus of Hsp90 and disrupting Hsp90-Cdc37 complex formation. Mol Cancer. 2014;13:150. doi: 10.1186/1476-4598-13-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Albakova Z, Mangasarova Y, Albakov A, Gorenkova L. HSP70 and HSP90 in Cancer: Cytosolic, Endoplasmic Reticulum and Mitochondrial Chaperones of Tumorigenesis. Front Oncol. 2022;12:829520. doi: 10.3389/fonc.2022.829520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qing G, Yan P, Xiao G. Hsp90 inhibition results in autophagy-mediated proteasome-independent degradation of IkappaB kinase (IKK) Cell Res. 2006;16:895–901. doi: 10.1038/sj.cr.7310109. [DOI] [PubMed] [Google Scholar]
- 39.Wang Q, Sun W, Hao X, Li T, Su L, Liu X. Down-regulation of cellular FLICE-inhibitory protein (Long Form) contributes to apoptosis induced by Hsp90 inhibition in human lung cancer cells. Cancer Cell Int. 2012;12:54. doi: 10.1186/1475-2867-12-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen WW, Yu H, Fan HB, Zhang CC, Zhang M, Zhang C, et al. RIP1 mediates the protection of geldanamycin on neuronal injury induced by oxygen-glucose deprivation combined with zVAD in primary cortical neurons. J Neurochem. 2012;120:70–7. doi: 10.1111/j.1471-4159.2011.07526.x. [DOI] [PubMed] [Google Scholar]
- 41.Li D, Li C, Li L, Chen S, Wang L, Li Q, et al. Natural Product Kongensin A is a Non-Canonical HSP90 Inhibitor that Blocks RIP3-dependent Necroptosis. Cell Chem Biol. 2016;23:257–66. doi: 10.1016/j.chembiol.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 42.Martini C, Bedard M, Lavigne P, Denault JB. Characterization of Hsp90 Co-Chaperone p23 Cleavage by Caspase-7 Uncovers a Peptidase-Substrate Interaction Involving Intrinsically Disordered Regions. Biochemistry. 2017;56:5099–111. doi: 10.1021/acs.biochem.7b00298. [DOI] [PubMed] [Google Scholar]
- 43.Kim JY, Han Y, Lee JE, Yenari MA. The 70-kDa heat shock protein (Hsp70) as a therapeutic target for stroke. Expert Opin Ther Targets. 2018;22:191–9. doi: 10.1080/14728222.2018.1439477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramos Rego I, Santos Cruz B, Ambrosio AF, Alves CH. TRAP1 in Oxidative Stress and Neurodegeneration. Antioxidants (Basel) 2021;10:1829. doi: 10.3390/antiox10111829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Birbo B, Madu EE, Madu CO, Jain A, Lu Y. Role of HSP90 in Cancer. Int J Mol Sci. 2021;22:10317. doi: 10.3390/ijms221910317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sumi MP, Ghosh A. Hsp90 in Human Diseases: Molecular Mechanisms to Therapeutic Approaches. Cells. 2022;11:976. doi: 10.3390/cells11060976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yin L, Yang Y, Zhu W, Xian Y, Han Z, Huang H, et al. Heat Shock Protein 90 Triggers Multi-Drug Resistance of Ovarian Cancer via AKT/GSK3beta/beta-Catenin Signaling. Front Oncol. 2021;11:620907. doi: 10.3389/fonc.2021.620907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yin XH, Han YL, Zhuang Y, Yan JZ, Li C. Geldanamycin inhibits Fas signaling pathway and protects neurons against ischemia. Neurosci Res. 2017;124:33–9. doi: 10.1016/j.neures.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 49.Niu M, Zhang B, Li L, Su Z, Pu W, Zhao C, et al. Targeting HSP90 Inhibits Proliferation and Induces Apoptosis Through AKT1/ERK Pathway in Lung Cancer. Front Pharm. 2021;12:724192. doi: 10.3389/fphar.2021.724192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dimas DT, Perlepe CD, Sergentanis TN, Misitzis I, Kontzoglou K, Patsouris E, et al. The Prognostic Significance of Hsp70/Hsp90 Expression in Breast Cancer: A Systematic Review and Meta-analysis. Anticancer Res. 2018;38:1551–62. doi: 10.21873/anticanres.12384. [DOI] [PubMed] [Google Scholar]
- 51.Cheng CJ, Liu KX, Zhang M, Shen FK, Ye LL, Wu WB, et al. Okicamelliaside targets the N-terminal chaperone pocket of HSP90 disrupts the chaperone protein interaction of HSP90-CDC37 and exerts antitumor activity. Acta Pharm Sin. 2022;43:1046–58. doi: 10.1038/s41401-021-00737-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang J, Cui S, Zhang X, Wu Y, Tang H. High expression of heat shock protein 90 is associated with tumor aggressiveness and poor prognosis in patients with advanced gastric cancer. PLoS One. 2013;8:e62876. doi: 10.1371/journal.pone.0062876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu K, Huang J, Liu J, Li C, Kroemer G, Tang D, et al. HSP90 Mediates IFNgamma-Induced Adaptive Resistance to Anti-PD-1 Immunotherapy. Cancer Res. 2022;82:2003–18. doi: 10.1158/0008-5472.CAN-21-3917. [DOI] [PubMed] [Google Scholar]
- 54.Ory B, Baud’huin M, Verrecchia F, Royer BB, Quillard T, Amiaud J, et al. Blocking HSP90 Addiction Inhibits Tumor Cell Proliferation, Metastasis Development, and Synergistically Acts with Zoledronic Acid to Delay Osteosarcoma Progression. Clin Cancer Res. 2016;22:2520–33. doi: 10.1158/1078-0432.CCR-15-1925. [DOI] [PubMed] [Google Scholar]
- 55.Li X, Tong X, Liu B, Li Z, Ding J, Li J, et al. Potential predictive value of plasma heat shock protein 90alpha in lung cancer. J Int Med Res. 2021;49:3000605211064393. doi: 10.1177/03000605211064393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sims JD, McCready J, Jay DG. Extracellular heat shock protein (Hsp)70 and Hsp90alpha assist in matrix metalloproteinase-2 activation and breast cancer cell migration and invasion. PLoS One. 2011;6:e18848. doi: 10.1371/journal.pone.0018848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mori M, Hitora T, Nakamura O, Yamagami Y, Horie R, Nishimura H, et al. Hsp90 inhibitor induces autophagy and apoptosis in osteosarcoma cells. Int J Oncol. 2015;46:47–54. doi: 10.3892/ijo.2014.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang Z, Li HM, Zhou C, Li Q, Ma L, Zhang Z, et al. Non-benzoquinone geldanamycin analogs trigger various forms of death in human breast cancer cells. J Exp Clin Cancer Res. 2016;35:149. doi: 10.1186/s13046-016-0428-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Song Q, Wen J, Li W, Xue J, Zhang Y, Liu H, et al. HSP90 promotes radioresistance of cervical cancer cells via reducing FBXO6-mediated CD147 polyubiquitination. Cancer Sci. 2022;113:1463–74. doi: 10.1111/cas.15269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang YQ, Shen AJ, Sun JY, Wang X, Liu HC, Zhang MM, et al. Targeting Hsp90 with FS-108 circumvents gefitinib resistance in EGFR mutant non-small cell lung cancer cells. Acta Pharm Sin. 2016;37:1587–96. doi: 10.1038/aps.2016.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sasame J, Ikegaya N, Kawazu M, Natsumeda M, Hayashi T, Isoda M, et al. HSP90 inhibition overcomes resistance to molecular targeted therapy in BRAFV600E mutant high-grade glioma. Clin Cancer Res. 2022;28:2425–39. doi: 10.1158/1078-0432.CCR-21-3622. [DOI] [PubMed] [Google Scholar]
- 62.Chen G, Cao P, Goeddel DV. TNF-induced recruitment and activation of the IKK complex require Cdc37 and Hsp90. Mol Cell. 2002;9:401–10. doi: 10.1016/s1097-2765(02)00450-1. [DOI] [PubMed] [Google Scholar]
- 63.Bai L, Xu S, Chen W, Li Z, Wang X, Tang H, et al. Blocking NF-kappaB and Akt by Hsp90 inhibition sensitizes Smac mimetic compound 3-induced extrinsic apoptosis pathway and results in synergistic cancer cell death. Apoptosis. 2011;16:45–54. doi: 10.1007/s10495-010-0542-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dutysheva EA, Utepova IA, Trestsova MA, Anisimov AS, Charushin VN, Chupakhin ON, et al. Synthesis and approbation of new neuroprotective chemicals of pyrrolyl- and indolylazine classes in a cell model of Alzheimer’s disease. Eur J Med Chem. 2021;222:113577. doi: 10.1016/j.ejmech.2021.113577. [DOI] [PubMed] [Google Scholar]
- 65.Shelton LB, Koren J, 3rd, Blair LJ. Imbalances in the Hsp90 Chaperone Machinery: Implications for Tauopathies. Front Neurosci. 2017;11:724. doi: 10.3389/fnins.2017.00724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen Y, Wang B, Liu D, Li JJ, Xue Y, Sakata K, et al. Hsp90 chaperone inhibitor 17-AAG attenuates Abeta-induced synaptic toxicity and memory impairment. J Neurosci. 2014;34:2464–70. doi: 10.1523/JNEUROSCI.0151-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rane A, Rajagopalan S, Ahuja M, Thomas B, Chinta SJ, Andersen JK. Hsp90 Co-chaperone p23 contributes to dopaminergic mitochondrial stress via stabilization of PHD2: Implications for Parkinson’s disease. Neurotoxicology. 2018;65:166–73. doi: 10.1016/j.neuro.2018.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Criado-Marrero M, Rein T, Binder EB, Porter JT, Koren J, 3rd, Blair LJ. Hsp90 and FKBP51: complex regulators of psychiatric diseases. Philos Trans R Soc Lond B Biol Sci. 2018;373:20160532. [DOI] [PMC free article] [PubMed]
- 69.Gassen NC, Hartmann J, Zschocke J, Stepan J, Hafner K, Zellner A, et al. Association of FKBP51 with priming of autophagy pathways and mediation of antidepressant treatment response: evidence in cells, mice, and humans. PLoS Med. 2014;11:e1001755. doi: 10.1371/journal.pmed.1001755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Peak SL, Gracia L, Lora G, Jinwal UK. Hsp90-interacting Co-chaperones and their Family Proteins in Tau Regulation: Introducing a Novel Role for Cdc37L1. Neuroscience. 2021;453:312–23. doi: 10.1016/j.neuroscience.2020.11.020. [DOI] [PubMed] [Google Scholar]
- 71.Bailus BJ, Scheeler SM, Simons J, Sanchez MA, Tshilenge KT, Creus-Muncunill J, et al. Modulating FKBP5/FKBP51 and autophagy lowers HTT (huntingtin) levels. Autophagy. 2021;17:4119–40. doi: 10.1080/15548627.2021.1904489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Criado-Marrero M, Gebru NT, Blazier DM, Gould LA, Baker JD, Beaulieu-Abdelahad D, et al. Hsp90 co-chaperones, FKBP52 and Aha1, promote tau pathogenesis in aged wild-type mice. Acta Neuropathol Commun. 2021;9:65. doi: 10.1186/s40478-021-01159-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Scotter EL, Chen HJ, Shaw CE. TDP-43 Proteinopathy and ALS: Insights into Disease Mechanisms and Therapeutic Targets. Neurotherapeutics. 2015;12:352–63. doi: 10.1007/s13311-015-0338-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lin LT-W, Razzaq A, Di Gregorio SE, Hong S, Charles B, Lopes MH, et al. Hsp90 and its co-chaperone Sti1 control TDP-43 misfolding and toxicity. FASEB J. 2020;35:e21594. [DOI] [PubMed]
- 75.Lackie RE, Maciejewski A, Ostapchenko VG, Marques-Lopes J, Choy WY, Duennwald ML, et al. The Hsp70/Hsp90 Chaperone Machinery in Neurodegenerative Diseases. Front Neurosci. 2017;11:254. doi: 10.3389/fnins.2017.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jinwal UK, Abisambra JF, Zhang J, Dharia S, O’Leary JC, Patel T, et al. Cdc37/Hsp90 protein complex disruption triggers an autophagic clearance cascade for TDP-43 protein. J Biol Chem. 2012;287:24814–20. doi: 10.1074/jbc.M112.367268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ghosh A, Chawla-Sarkar M, Stuehr DJ. Hsp90 interacts with inducible NO synthase client protein in its heme-free state and then drives heme insertion by an ATP-dependent process. FASEB J. 2011;25:2049–60. doi: 10.1096/fj.10-180554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Aceros H, Der Sarkissian S, Borie M, Stevens LM, Mansour S, Noiseux N. Celastrol-type HSP90 modulators allow for potent cardioprotective effects. Life Sci. 2019;227:8–19. doi: 10.1016/j.lfs.2019.04.025. [DOI] [PubMed] [Google Scholar]
- 79.Lam CK, Zhao W, Cai W, Vafiadaki E, Florea SM, Ren X, et al. Novel role of HAX-1 in ischemic injury protection involvement of heat shock protein 90. Circ Res. 2013;112:79–89. doi: 10.1161/CIRCRESAHA.112.279935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang DX, Huang Z, Li QJ, Zhong GQ, He Y, Huang WQ, et al. Involvement of HSP90 in ischemic postconditioning-induced cardioprotection by inhibition of the complement system, JNK and inflammation. Acta Cir Bras. 2020;35:e202000105. doi: 10.1590/s0102-865020200010000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang E, Chen Q, Wang J, Li D, Wan Z, Ju X. Protective role of microRNA-27a upregulation and HSP90 silencing against cerebral ischemia-reperfusion injury in rats by activating PI3K/AKT/mTOR signaling pathway. Int Immunopharmacol. 2020;86:106635. doi: 10.1016/j.intimp.2020.106635. [DOI] [PubMed] [Google Scholar]
- 82.Lu A, Ran R, Parmentier-Batteur S, Nee A, Sharp FR. Geldanamycin induces heat shock proteins in brain and protects against focal cerebral ischemia. J Neurochem. 2002;81:355–64. doi: 10.1046/j.1471-4159.2002.00835.x. [DOI] [PubMed] [Google Scholar]
- 83.Wen XR, Li C, Zong YY, Yu CZ, Xu J, Han D, et al. Dual inhibitory roles of geldanamycin on the c-Jun NH2-terminal kinase 3 signal pathway through suppressing the expression of mixed-lineage kinase 3 and attenuating the activation of apoptosis signal-regulating kinase 1 via facilitating the activation of Akt in ischemic brain injury. Neuroscience. 2008;156:483–97. doi: 10.1016/j.neuroscience.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 84.Uddin MA, Akhter MS, Kubra KT, Barabutis N. Hsp90 inhibition protects brain endothelial cells against LPS-induced injury. Biofactors. 2022;48:926–33. [DOI] [PMC free article] [PubMed]
- 85.Qi J, Liu Y, Yang P, Chen T, Liu XZ, Yin Y, et al. Heat shock protein 90 inhibition by 17-Dimethylaminoethylamino-17-demethoxygeldanamycin protects blood-brain barrier integrity in cerebral ischemic stroke. Am J Transl Res. 2015;7:1826–37. [PMC free article] [PubMed] [Google Scholar]
- 86.Pezzulo AA, Tudas RA, Stewart CG, Buonfiglio LGV, Lindsay BD, Taft PJ, et al. HSP90 inhibitor geldanamycin reverts IL-13- and IL-17-induced airway goblet cell metaplasia. J Clin Invest. 2019;129:744–58. doi: 10.1172/JCI123524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lisi L, McGuire S, Sharp A, Chiosis G, Navarra P, Feinstein DL, et al. The novel HSP90 inhibitor, PU-H71, suppresses glial cell activation but weakly affects clinical signs of EAE. J Neuroimmunol. 2013;255:1–7. doi: 10.1016/j.jneuroim.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nascimento DSM, Potes CS, Soares ML, Ferreira AC, Malcangio M, Castro-Lopes JM, et al. Drug-Induced HSP90 Inhibition Alleviates Pain in Monoarthritic Rats and Alters the Expression of New Putative Pain Players at the DRG. Mol Neurobiol. 2018;55:3959–75. doi: 10.1007/s12035-017-0628-x. [DOI] [PubMed] [Google Scholar]
- 89.Akhter MS, Uddin MA, Kubra KT, Barabutis N. Elucidation of the Molecular Pathways Involved in the Protective Effects of AUY-922 in LPS-Induced Inflammation in Mouse Lungs. Pharmaceuticals (Basel). 2021;14:522. [DOI] [PMC free article] [PubMed]
- 90.Jayaprakash P, Dong H, Zou M, Bhatia A, O'Brien K, Chen M, et al Hsp90α and Hsp90β together operate a hypoxia and nutrient paucity stress-response mechanism during wound healing. J Cell Sci. 2015;128:1475–80. [DOI] [PMC free article] [PubMed]
- 91.Yang Y, Sangwung P, Kondo R, Jung Y, McConnell MJ, Jeong J, et al. Alcohol-induced Hsp90 acetylation is a novel driver of liver sinusoidal endothelial dysfunction and alcohol-related liver disease. J Hepatol. 2021;75:377–86. doi: 10.1016/j.jhep.2021.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zaman S, Wang R, Gandhi V. Targeting the apoptosis pathway in hematologic malignancies. Leuk Lymphoma. 2014;55:1980–92. doi: 10.3109/10428194.2013.855307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bertheloot D, Latz E, Franklin BS. Necroptosis, pyroptosis and apoptosis: an intricate game of cell death. Cell Mol Immunol. 2021;18:1106–21. doi: 10.1038/s41423-020-00630-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang X, Ju W, Renouard J, Aden J, Belinsky SA, Lin Y. 17-allylamino-17-demethoxygeldanamycin synergistically potentiates tumor necrosis factor-induced lung cancer cell death by blocking the nuclear factor-kappaB pathway. Cancer Res. 2006;66:1089–95. doi: 10.1158/0008-5472.CAN-05-2698. [DOI] [PubMed] [Google Scholar]
- 96.Nimmanapalli R, O’Bryan E, Kuhn D, Yamaguchi H, Wang HG, Bhalla KN. Regulation of 17-AAG-induced apoptosis: role of Bcl-2, Bcl-XL, and Bax downstream of 17-AAG-mediated down-regulation of Akt, Raf-1, and Src kinases. Blood. 2003;102:269–75. doi: 10.1182/blood-2002-12-3718. [DOI] [PubMed] [Google Scholar]
- 97.Yan C, Oh JS, Yoo SH, Lee JS, Yoon YG, Oh YJ, et al. The targeted inhibition of mitochondrial Hsp90 overcomes the apoptosis resistance conferred by Bcl-2 in Hep3B cells via necroptosis. Toxicol Appl Pharm. 2013;266:9–18. doi: 10.1016/j.taap.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 98.Kim YJ, Lee SA, Myung SC, Kim W, Lee CS. Radicicol, an inhibitor of Hsp90, enhances TRAIL-induced apoptosis in human epithelial ovarian carcinoma cells by promoting activation of apoptosis-related proteins. Mol Cell Biochem. 2012;359:33–43. doi: 10.1007/s11010-011-0997-9. [DOI] [PubMed] [Google Scholar]
- 99.Pandey P, Saleh A, Nakazawa A, Kumar S, Srinivasula SM, Kumar V, et al. Negative regulation of cytochrome c-mediated oligomerization of Apaf-1 and activation of procaspase-9 by heat shock protein 90. EMBO J. 2000;19:4310–22. doi: 10.1093/emboj/19.16.4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhao X, Wang J, Xiao L, Xu Q, Zhao E, Zheng X, et al. Effects of 17-AAG on the cell cycle and apoptosis of H446 cells and the associated mechanisms. Mol Med Rep. 2016;14:1067–74. doi: 10.3892/mmr.2016.5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shen CH, Hsieh CC, Jiang KY, Lin CY, Chiang NJ, Li TW, et al. AUY922 induces retinal toxicity through attenuating TRPM1. J Biomed Sci. 2021;28:55. doi: 10.1186/s12929-021-00751-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mimura N, Fulciniti M, Gorgun G, Tai YT, Cirstea D, Santo L, et al. Blockade of XBP1 splicing by inhibition of IRE1alpha is a promising therapeutic option in multiple myeloma. Blood. 2012;119:5772–81. doi: 10.1182/blood-2011-07-366633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Woo SM, Seo SU, Kubatka P, Min KJ, Kwon TK. Honokiol Enhances TRAIL-Mediated Apoptosis through STAMBPL1-Induced Survivin and c-FLIP Degradation. Biomolecules. 2019;9:838. doi: 10.3390/biom9120838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ranek MJ, Oeing C, Sanchez-Hodge R, Kokkonen-Simon KM, Dillard D, Aslam MI, et al. CHIP phosphorylation by protein kinase G enhances protein quality control and attenuates cardiac ischemic injury. Nat Commun. 2020;11:5237. doi: 10.1038/s41467-020-18980-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Park MA, Zhang G, Mitchell C, Rahmani M, Hamed H, Hagan MP, et al. Mitogen-activated protein kinase kinase 1/2 inhibitors and 17-allylamino-17-demethoxygeldanamycin synergize to kill human gastrointestinal tumor cells in vitro via suppression of c-FLIP-s levels and activation of CD95. Mol Cancer Ther. 2008;7:2633–48. doi: 10.1158/1535-7163.MCT-08-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J Pathol. 2010;221:3–12. doi: 10.1002/path.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li W, Nie T, Xu H, Yang J, Yang Q, Mao Z. Chaperone-mediated autophagy: Advances from bench to bedside. Neurobiol Dis. 2019;122:41–8. doi: 10.1016/j.nbd.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 108.Bandyopadhyay U, Kaushik S, Varticovski L, Cuervo AM. The chaperone-mediated autophagy receptor organizes in dynamic protein complexes at the lysosomal membrane. Mol Cell Biol. 2008;28:5747–63. doi: 10.1128/MCB.02070-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kimura T, Uesugi M, Takase K, Miyamoto N, Sawada K. Hsp90 inhibitor geldanamycin attenuates the cytotoxicity of sunitinib in cardiomyocytes via inhibition of the autophagy pathway. Toxicol Appl Pharm. 2017;329:282–92. doi: 10.1016/j.taap.2017.06.015. [DOI] [PubMed] [Google Scholar]
- 110.Joo JH, Dorsey FC, Joshi A, Hennessy-Walters KM, Rose KL, McCastlain K, et al. Hsp90-Cdc37 chaperone complex regulates Ulk1- and Atg13-mediated mitophagy. Mol Cell. 2011;43:572–85. doi: 10.1016/j.molcel.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zachari M, Ganley IG. The mammalian ULK1 complex and autophagy initiation. Essays Biochem. 2017;61:585–96. doi: 10.1042/EBC20170021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Han J, Goldstein LA, Hou W, Chatterjee S, Burns TF, Rabinowich H. HSP90 inhibition targets autophagy and induces a CASP9-dependent resistance mechanism in NSCLC. Autophagy. 2018;14:958–71. doi: 10.1080/15548627.2018.1434471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang B, Chen Z, Yu F, Chen Q, Tian Y, Ma S, et al. Hsp90 regulates autophagy and plays a role in cancer therapy. Tumour Biol. 2016;37:1–6. doi: 10.1007/s13277-015-4142-3. [DOI] [PubMed] [Google Scholar]
- 114.Xu C, Liu J, Hsu LC, Luo Y, Xiang R, Chuang TH. Functional interaction of heat shock protein 90 and Beclin 1 modulates Toll-like receptor-mediated autophagy. FASEB J. 2011;25:2700–10. doi: 10.1096/fj.10-167676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liu KS, Liu H, Qi JH, Liu QY, Liu Z, Xia M, et al. SNX-2112, an Hsp90 inhibitor, induces apoptosis and autophagy via degradation of Hsp90 client proteins in human melanoma A-375 cells. Cancer Lett. 2012;318:180–8. doi: 10.1016/j.canlet.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 116.He W, Ye X, Huang X, Lel W, You L, Wang L, et al. Hsp90 inhibitor, BIIB021, induces apoptosis and autophagy by regulating mTOR-Ulk1 pathway in imatinib-sensitive and -resistant chronic myeloid leukemia cells. Int J Oncol. 2016;48:1710–20. doi: 10.3892/ijo.2016.3382. [DOI] [PubMed] [Google Scholar]
- 117.Hu B, Zhang Y, Jia L, Wu H, Fan C, Sun Y, et al. Binding of the pathogen receptor HSP90AA1 to avibirnavirus VP2 induces autophagy by inactivating the AKT-MTOR pathway. Autophagy. 2015;11:503–15. doi: 10.1080/15548627.2015.1017184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Marunouchi T, Nishiumi C, Iinuma S, Yano E, Tanonaka K. Effects of Hsp90 inhibitor on the RIP1-RIP3-MLKL pathway during the development of heart failure in mice. Eur J Pharm. 2021;898:173987. doi: 10.1016/j.ejphar.2021.173987. [DOI] [PubMed] [Google Scholar]
- 119.Li D, Xu T, Cao Y, Wang H, Li L, Chen S, et al. A cytosolic heat shock protein 90 and cochaperone CDC37 complex is required for RIP3 activation during necroptosis. Proc Natl Acad Sci USA. 2015;112:5017–22. doi: 10.1073/pnas.1505244112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yu X, Mao M, Liu X, Shen T, Li T, Yu H, et al. A cytosolic heat shock protein 90 and co-chaperone p23 complex activates RIPK3/MLKL during necroptosis of endothelial cells in acute respiratory distress syndrome. J Mol Med (Berl) 2020;98:569–83. doi: 10.1007/s00109-020-01886-y. [DOI] [PubMed] [Google Scholar]
- 121.Wang Y, Ma H, Huang J, Yao Z, Yu J, Zhang W, et al. Discovery of bardoxolone derivatives as novel orally active necroptosis inhibitors. Eur J Med Chem. 2021;212:113030. doi: 10.1016/j.ejmech.2020.113030. [DOI] [PubMed] [Google Scholar]
- 122.Zhao XM, Chen Z, Zhao JB, Zhang PP, Pu YF, Jiang SH, et al. Hsp90 modulates the stability of MLKL and is required for TNF-induced necroptosis. Cell Death Dis. 2016;7:e2089. doi: 10.1038/cddis.2015.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dondelinger Y, Declercq W, Montessuit S, Roelandt R, Goncalves A, Bruggeman I, et al. MLKL compromises plasma membrane integrity by binding to phosphatidylinositol phosphates. Cell Rep. 2014;7:971–81. doi: 10.1016/j.celrep.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 124.Jacobsen AV, Lowes KN, Tanzer MC, Lucet IS, Hildebrand JM, Petrie EJ, et al. HSP90 activity is required for MLKL oligomerisation and membrane translocation and the induction of necroptotic cell death. Cell Death Dis. 2016;7:e2051. doi: 10.1038/cddis.2015.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Su X, Cao Y, Liu Y, Ouyang B, Ning B, Wang Y, et al. Localized disruption of redox homeostasis boosting ferroptosis of tumor by hydrogel delivery system. Mater Today Bio. 2021;12:100154. doi: 10.1016/j.mtbio.2021.100154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Forcina GC, Dixon SJ. GPX4 at the Crossroads of Lipid Homeostasis and Ferroptosis. Proteomics. 2019;19:e1800311. doi: 10.1002/pmic.201800311. [DOI] [PubMed] [Google Scholar]
- 127.Cuervo AM, Dice JF. A receptor for the selective uptake and degradation of proteins by lysosomes. Science. 1996;273:501–3. doi: 10.1126/science.273.5274.501. [DOI] [PubMed] [Google Scholar]
- 128.Yu S, Li Z, Zhang Q, Wang R, Zhao Z, Ding W, et al. GPX4 degradation via chaperone-mediated autophagy contributes to antimony-triggered neuronal ferroptosis. Ecotoxicol Environ Saf. 2022;234:113413. doi: 10.1016/j.ecoenv.2022.113413. [DOI] [PubMed] [Google Scholar]
- 129.Miao Z, Tian W, Ye Y, Gu W, Bao Z, Xu L, et al. Hsp90 induces Acsl4-dependent glioma ferroptosis via dephosphorylating Ser637 at Drp1. Cell Death Dis. 2022;13:548. doi: 10.1038/s41419-022-04997-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yu P, Zhang X, Liu N, Tang L, Peng C, Chen X. Pyroptosis: mechanisms and diseases. Signal Transduct Target Ther. 2021;6:128. doi: 10.1038/s41392-021-00507-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Garcia-Carbonero R, Carnero A, Paz-Ares L. Inhibition of HSP90 molecular chaperones: moving into the clinic. Lancet Oncol. 2013;14:e358–e69. doi: 10.1016/S1470-2045(13)70169-4. [DOI] [PubMed] [Google Scholar]
- 132.Piippo N, Korhonen E, Hytti M, Skottman H, Kinnunen K, Josifovska N, et al. Hsp90 inhibition as a means to inhibit activation of the NLRP3 inflammasome. Sci Rep. 2018;8:6720. doi: 10.1038/s41598-018-25123-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Vanden Berghe T, Kalai M, van Loo G, Declercq W, Vandenabeele P. Disruption of HSP90 function reverts tumor necrosis factor-induced necrosis to apoptosis. J Biol Chem. 2003;278:5622–9. doi: 10.1074/jbc.M208925200. [DOI] [PubMed] [Google Scholar]
- 134.D’Arcy MS. Cell death: a review of the major forms of apoptosis, necrosis and autophagy. Cell Biol Int. 2019;43:582–92. doi: 10.1002/cbin.11137. [DOI] [PubMed] [Google Scholar]
- 135.Ward GA, Lewis EJ, Ahn JS, Johnson CN, Lyons JF, Martins V, et al. ASTX660, a Novel Non-peptidomimetic Antagonist of cIAP1/2 and XIAP, Potently Induces TNFalpha-Dependent Apoptosis in Cancer Cell Lines and Inhibits Tumor Growth. Mol Cancer Ther. 2018;17:1381–91. doi: 10.1158/1535-7163.MCT-17-0848. [DOI] [PubMed] [Google Scholar]
- 136.Lafont E, Kantari-Mimoun C, Draber P, De Miguel D, Hartwig T, Reichert M, et al. The linear ubiquitin chain assembly complex regulates TRAIL-induced gene activation and cell death. EMBO J. 2017;36:1147–66. doi: 10.15252/embj.201695699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gordy C, He YW. The crosstalk between autophagy and apoptosis: where does this lead? Protein Cell. 2012;3:17–27. doi: 10.1007/s13238-011-1127-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Song X, Zhu S, Chen P, Hou W, Wen Q, Liu J, et al. AMPK-Mediated BECN1 Phosphorylation Promotes Ferroptosis by Directly Blocking System Xc(-) Activity. Curr Biol. 2018;28:2388–99.e5. doi: 10.1016/j.cub.2018.05.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chen G, Zhang X, Zhao M, Wang Y, Cheng X, Wang D, et al. Celastrol targets mitochondrial respiratory chain complex I to induce reactive oxygen species-dependent cytotoxicity in tumor cells. BMC Cancer. 2011;11:170. doi: 10.1186/1471-2407-11-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Basit F, van Oppen LM, Schockel L, Bossenbroek HM. van Emst-de Vries SE, Hermeling JC, et al. Mitochondrial complex I inhibition triggers a mitophagy-dependent ROS increase leading to necroptosis and ferroptosis in melanoma cells. Cell Death Dis. 2017;8:e2716. doi: 10.1038/cddis.2017.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Henry CM, Martin SJ. Caspase-8 Acts in a Non-enzymatic Role as a Scaffold for Assembly of a Pro-inflammatory “FADDosome” Complex upon TRAIL Stimulation. Mol Cell. 2017;65:715–29 e5. doi: 10.1016/j.molcel.2017.01.022. [DOI] [PubMed] [Google Scholar]
- 142.Jeong HS, Choi HY, Lee ER, Kim JH, Jeon K, Lee HJ, et al. Involvement of caspase-9 in autophagy-mediated cell survival pathway. Biochim Biophys Acta. 2011;1813:80–90. doi: 10.1016/j.bbamcr.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 143.Chaudhury S, Narasimharao Meka P, Banerjee M, Kent CN, Blagg BSJ. Structure-Based Design, Synthesis, and Biological Evaluation of Hsp90beta-Selective Inhibitors. Chemistry. 2021;27:14747–64. doi: 10.1002/chem.202102574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Abdelmoaty AAA, Zhang P, Lin W, Fan YJ, Ye SN, Xu JH. C0818, a novel curcumin derivative, induces ROS-dependent cytotoxicity in human hepatocellular carcinoma cells in vitro via disruption of Hsp90 function. Acta Pharm Sin. 2022;43:446–56. doi: 10.1038/s41401-021-00642-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xue N, Lai F, Du T, Ji M, Liu D, Yan C, et al. Chaperone-mediated autophagy degradation of IGF-1Rbeta induced by NVP-AUY922 in pancreatic cancer. Cell Mol Life Sci. 2019;76:3433–47. doi: 10.1007/s00018-019-03080-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hertlein E, Wagner AJ, Jones J, Lin TS, Maddocks KJ, Towns WH, 3rd, et al. 17-DMAG targets the nuclear factor-kappaB family of proteins to induce apoptosis in chronic lymphocytic leukemia: clinical implications of HSP90 inhibition. Blood. 2010;116:45–53. doi: 10.1182/blood-2010-01-263756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Aceros H, Der Sarkissian S, Borie M, Pinto Ribeiro RV, Maltais S, Stevens LM, et al. Novel heat shock protein 90 inhibitor improves cardiac recovery in a rodent model of donation after circulatory death. J Thorac Cardiovasc Surg. 2022;163:e187–e97. doi: 10.1016/j.jtcvs.2020.03.042. [DOI] [PubMed] [Google Scholar]
- 148.Chen B, Yang B, Zhu J, Wu J, Sha J, Sun J, et al. Hsp90 Relieves Heat Stress-Induced Damage in Mouse Kidneys: Involvement of Antiapoptotic PKM2-AKT and Autophagic HIF-1alpha Signaling. Int J Mol Sci. 2020;21:1646. doi: 10.3390/ijms21051646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chen F, Xie H, Bao H, Violetta L, Zheng S. Combination of HSP90 and autophagy inhibitors promotes hepatocellular carcinoma apoptosis following incomplete thermal ablation. Mol Med Rep. 2020;22:337–43. doi: 10.3892/mmr.2020.11080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Xu G, Ma X, Chen F, Wu D, Miao J, Fan Y. 17-DMAG disrupted the autophagy flux leading to the apoptosis of acute lymphoblastic leukemia cells by inducing heat shock cognate protein 70. Life Sci. 2020;249:117532. doi: 10.1016/j.lfs.2020.117532. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Original data used for this report (although not applicable) will be made available upon request to the corresponding author.