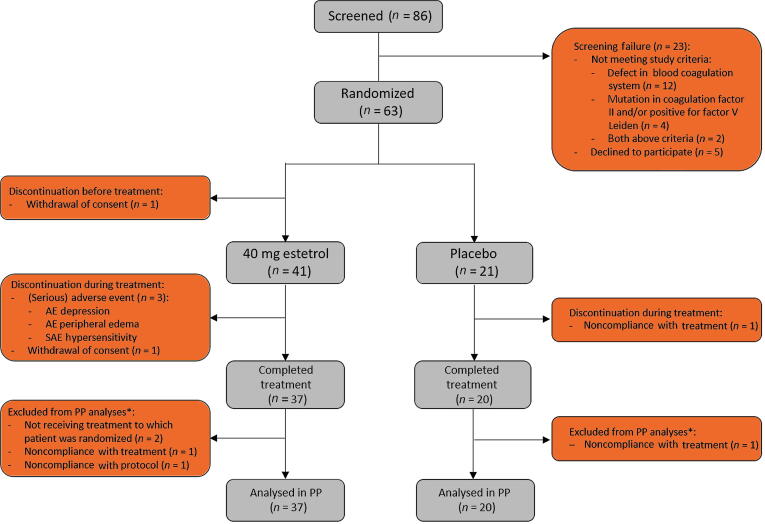
Fig. 1.
CONSORT diagram. PP = per protocol. *Based on all patients who started treatment; patients not completing the study treatment could still be included in the PP analyses if all study assessments were performed (eg, end-of-study visit). This was applicable for the patients in the estetrol group, but not for the patients in the placebo group. All patients who received at least one dose of study medication were included in analyses of the safety parameters.