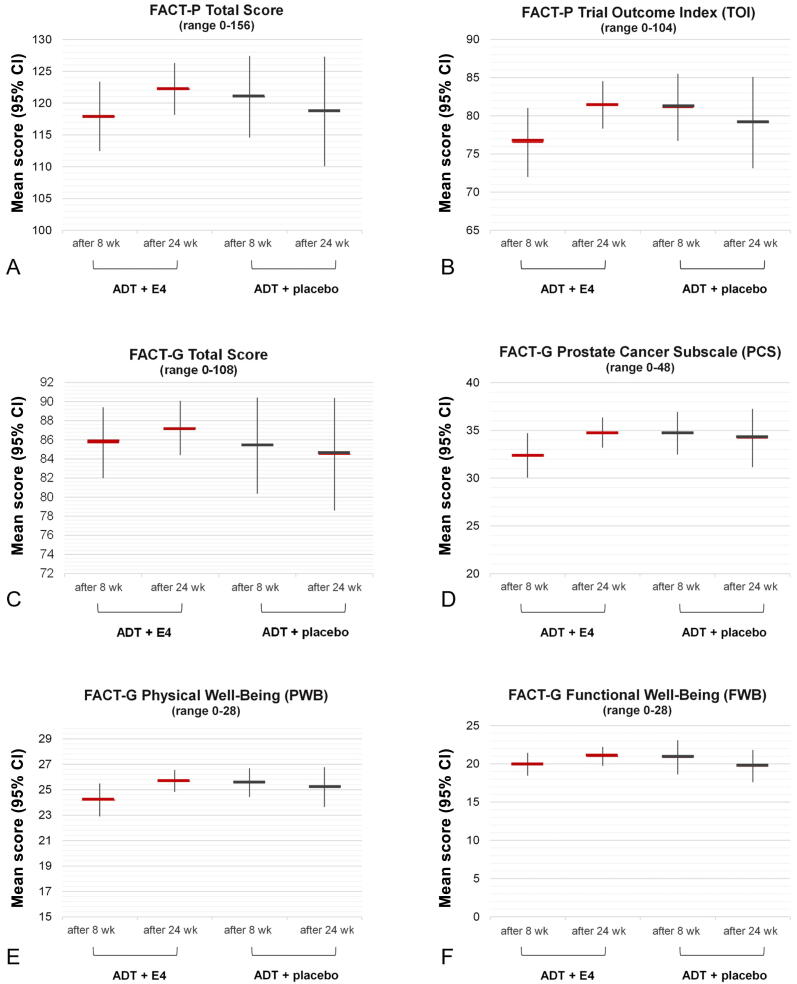
Fig. 3.
Mean scores for (A) FACT-P Total (A), (B) FACT-P Trial Outcome Index (TOI), (C) FACT-G Total, (D), FACT-G Prostate Cancer Subscale (PCS), (E) FACT-G Physical Well-Being (PWB), and (F) FACT-G Functional Well-Being (FWB) domains after 8 wk and 24 wk of treatment with 40 mg of estetrol (E4) or placebo in patients with prostate cancer treated with a luteinizing hormone–releasing hormone agonist (per-protocol population). FACT-P Total score = sum of PWB score + SWB score + EWB score + FWB score + PCS score. FACT-P TOI score = sum of PWB score + FWB score + PCS score; FACT-G Total score = sum of PWB score + SWB score + EWB score + FWB score. Higher scores correspond to better quality of life. ADT = androgen deprivation therapy; CI = confidence interval; E4 = estetrol; FACT-G = Functional Assessment of Cancer Therapy-General; FACT-P = Functional Assessment of Cancer Therapy-Prostate.