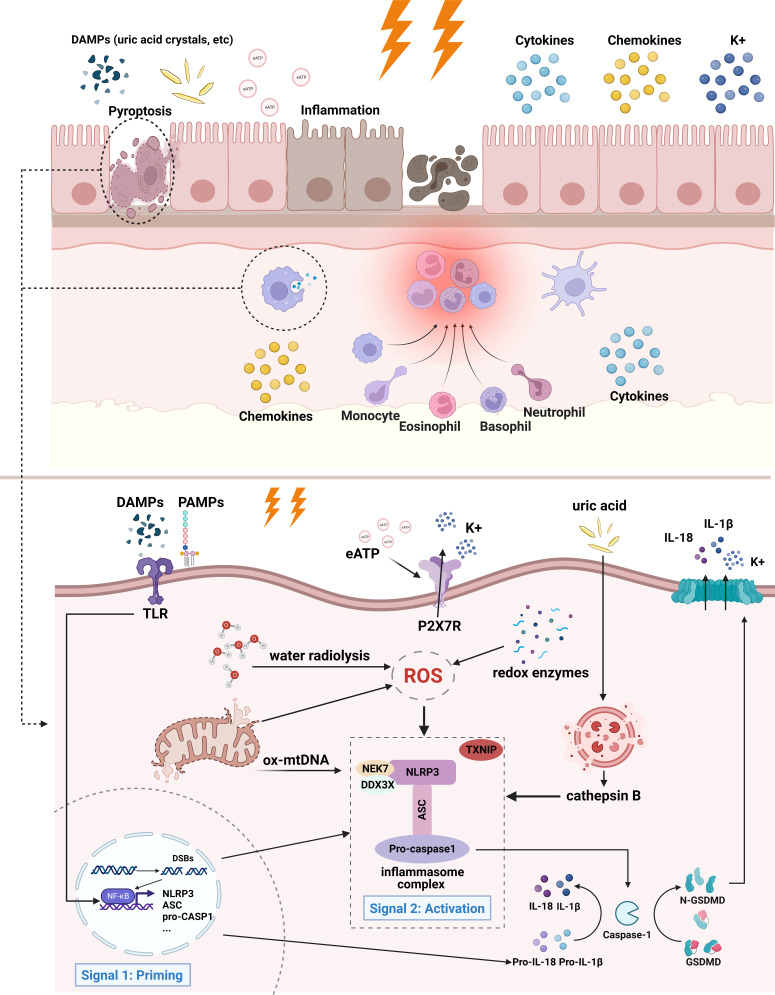
Figure 1.
Mechanism of radiation-induced NLRP3 inflammasome activation. In the case of intestinal epithelial cells and infiltrating macrophages, irradiated cells initiate inflammatory responses and eventually undergo cell death. Adjacent cells may also be affected by inflammation. Specifically, extracellular PAMPs and DAMPs act as priming signals, binding to and inducing TLR signaling, thereby promoting the activation of NF-κB and the transcription of inflammasome components and pro-IL-1β. The activation signals entail ROS, K+ efflux, release of mitochondrial DNA, lysosomal rupture and cathepsin release, etc. ROS is produced from the radiolysis of water, redox enzymes, and dysfunctional mitochondria. Lysosomal membrane may be destabilized by particulate or crystalline structures (like uric acid crystal) and then lead to lysosomal rupture, releasing cathepsin B. Mitochondrial dysfunction gives rise to the release of mtDNA and cardiolipin. These activation signals stimulate NLRP3 inflammasome and lead to its oligomerization. Activated NLRP3 inflammasome mediates the maturation of IL-1β and IL-18, as well as induces pyroptosis by cleaving GSDMD. The downstream effects of NLRP3 inflammasome activation are characterized by the production of cytokines, chemokines, and recruitment of immune cells, followed by cell death. Of notes, the contribution of mtDNA and extracellular ATP to radiation-induced inflammasome has not yet been confirmed, therefore they are illustrated with fading arrows.