Figure 5.
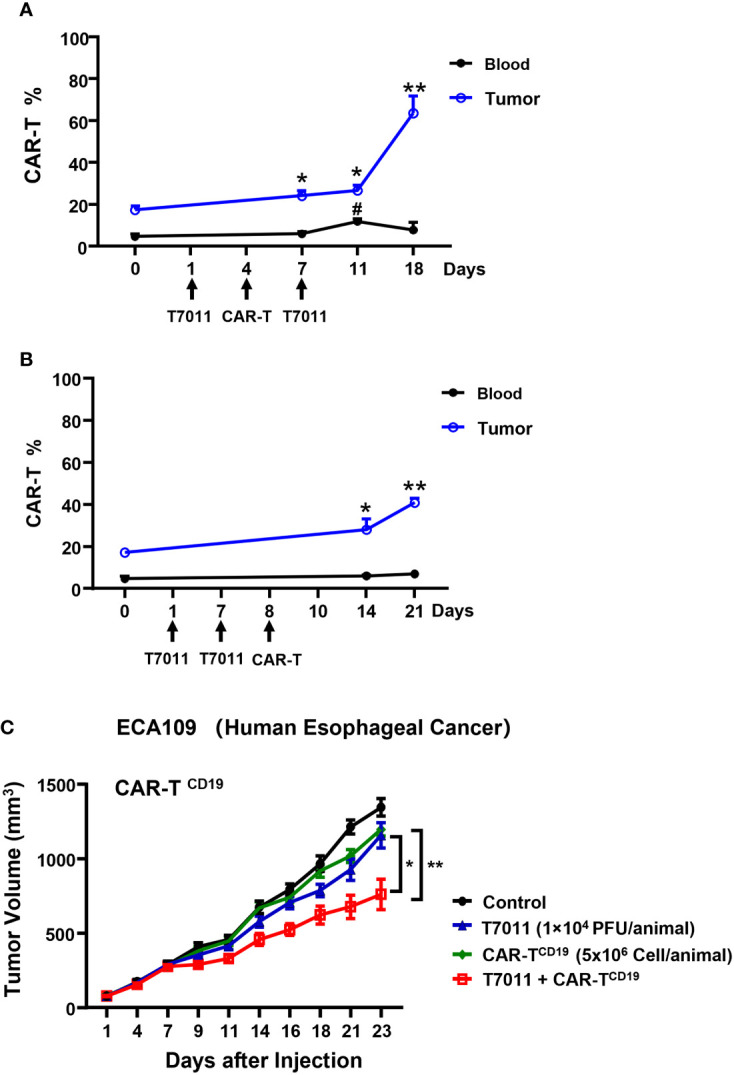
Tumor antigen delivered by T7011 directly activates CAR T cells and sensitizes antitumor activity of CD19-specific CAR T cells against solid tumor cells in human xenograft tumor model. (A, B) Percentage of CAR T cells in the blood and tumor tissues in combination with T7011 with different administration sequences. Mice were engrafted with subcutaneous (s.c.) ECA109 tumor cells (3×106 cells), 12 days after inoculation, mice (n=3 per group) were intratumorally (i.t.) injected with T7011 (104 PFU per mouse) on D1, D7, and intravenously (i.v.) injected with CD19-specific CAR T cells (CAR-TCD19, 5 × 106 cells) on D8 (A) or D4 (B). Tumor tissues and blood were harvested at D0 before dosing or indicated time-points after the first treatment with T7011. The CAR T cells were quantified by flow cytometry as described in Methods and Materials. The data are expressed as the mean percentage of human CD3 positive T cells in total viable cells. Statistically significant differences are indicated by asterisks in tumor and by pound signs in blood. (*p < 0.05; **p < 0.01, #p < 0.05). (C) Antitumor efficacy of combination therapy of T7011 and CD19-specific CAR T cells in subcutaneous ECA109 tumors model. Mice were engrafted with ECA109 tumors (3×106 cells) subcutaneously (s.c.), 12 days after inoculation, the tumor volume reached 80 mm3 were intratumorally (i.t.) injected with T7011 (104 PFU per mouse) on D1 and D7 and intravenously (i.v.) injected with CD19-specific CAR T cells (CAR-TCD19, 5 × 106 cells) on D4. Tumor volumes are shown as means ± SEM (n =8 per group). Statistically significant differences are indicated by asterisks (*p < 0.05, **p < 0.01).
