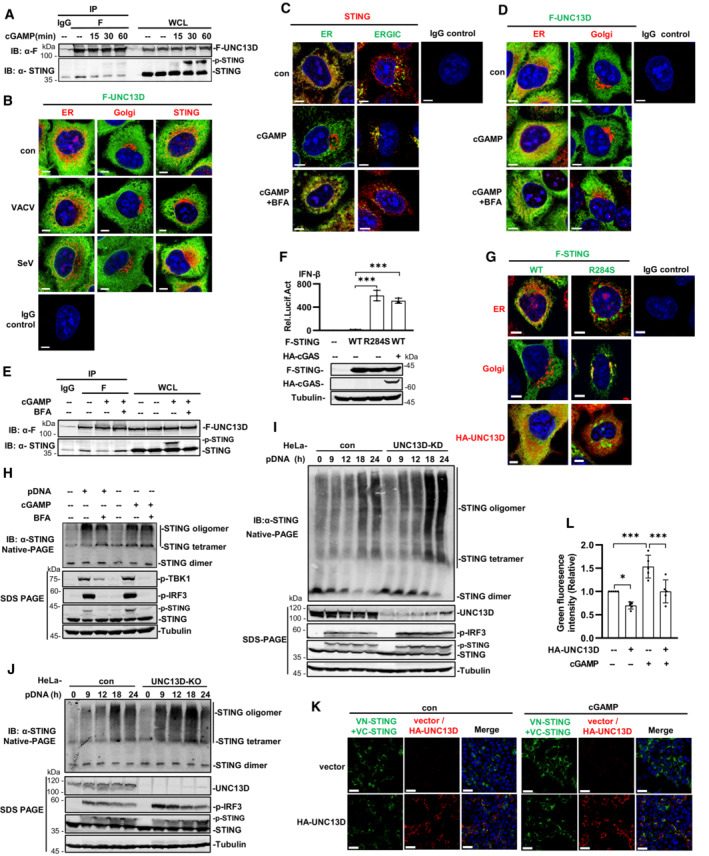
Figure 3. UNC13D inhibits STING oligomerization on the endoplasmic reticulum.
-
AUNC13D knockout HeLa cells stably expressing FLAG‐tagged UNC13D were stimulated with cGAMP (100 nM) or left unstimulated for the indicated time. Whole cell lysates (WCL) were examined, and cell lysates were immunoprecipitated (IP) with IgG and anti‐Flag antibodies, followed by immunoblotting (IB) with the indicated antibodies.
-
BUNC13D knockout HeLa cells stably expressing FLAG‐tagged UNC13D were infected with VACV or SeV for 12 h. The localization of FLAG‐UNC13D (green), ER marker calnexin (red), Golgi marker GM130 (red), endogenous STING (red) and nuclei (blue) was examined via confocal microscopy. Scale bar: 6 μm.
-
C–EWild‐type HeLa cells (C) and UNC13D knockout HeLa cells stably expressing FLAG‐tagged UNC13D (D and E) were pretreated with BFA (20 μM) or left untreated for 2 h, followed by stimulation with cGAMP (100 nM) (or left unstimulated) for 30 min. Localization of endogenous STING (red), FLAG‐UNC13D (green), ER marker calnexin (red/green), ERGIC maker ERGIC‐53 (green), Golgi marker GM130 (red) and nuclei (blue) was examined via confocal microscopy. Scale bar: 6 μm (C and D). Whole cell lysates (WCL) were examined, and cell lysates were immunoprecipitated (IP) with IgG and anti‐Flag antibodies, followed by immunoblotting (IB) with the indicated antibodies (E).
-
FHEK293T cells were transfected for 24 h with IFN‐β‐luciferase reporter plasmid, pRL‐TK and the indicated expression plasmids, followed by dual‐luciferase reporter assays. The expression of plasmids was examined by western blotting with anti‐Flag, ‐HA or ‐tubulin antibody. n = 3 technical replicates.
-
GSTING knockout HeLa cells were transfected with FLAG‐tagged STING WT/R284S (1 μg), HA‐tagged UNC13D (1 μg) or vector (1 μg) for 24 h. The localization of FLAG‐STING WT or R284S (green), endogenous ER marker calnexin (red), Golgi marker GM130 (red), HA‐UNC13D (red) and nuclei (blue) was examined via confocal microscopy. Scale bar: 5 μm.
-
HWild‐type HeLa cells were pretreated with BFA (20 μM) or left untreated for 2 h before stimulation with plasmid DNA (1 μg/ml) for 18 h or cGAMP (100 nM) for 30 min (or left unstimulated). Cell lysates were separated by native (top) or SDS (bottom) PAGE and analyzed by immunoblotting (IB) with anti‐p‐TBK1, ‐p‐IRF3, ‐STING or ‐tubulin antibody.
-
I, JUNC13D stable knockdown (KD) (I) and knockout (KO) (J) HeLa cells and control cells were transfected with plasmid DNA (pDNA) (1 μg/ml) for the indicated time. Cell lysates were separated by native (top) or SDS (bottom) PAGE and analyzed by immunoblotting (IB) with anti‐UNC13D, ‐p‐TBK1, ‐p‐IRF3, ‐STING or ‐tubulin antibody.
-
K, LHEK293T cells were transfected with HA‐tagged UNC13D, VN/VC‐STING or vector for 24 h, followed by stimulation with cGAMP (100 nM) (or left unstimulated) for 30 min. Nucleus marker DAPI (blue), VN/VC‐STING (green) and HA‐UNC13D (red) were examined via confocal microscopy (K). Scale bar: 25 μm. The green fluorescence intensity was determined by ImageJ (L). n = 5 biological replicates.
Data information: Statistically significant differences were determined using ANOVA. Data are shown as the mean ± s.d. ns, not significant, P > 0.05; *P < 0.05; ***P < 0.001.
Source data are available online for this figure.
