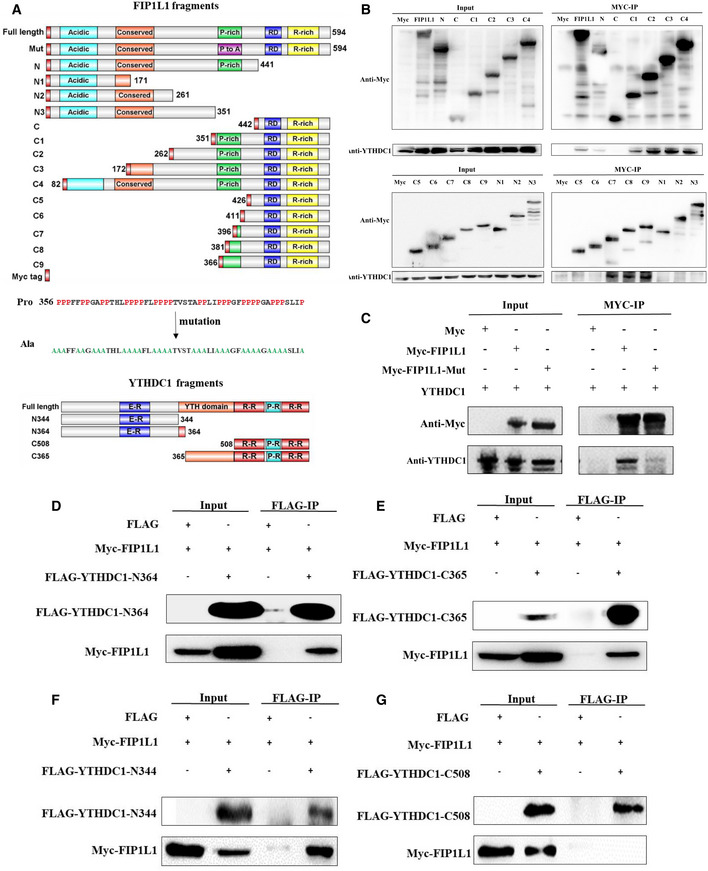
Figure 5. Proline‐rich domain of FIP1L1 is necessary for the interaction with YTHDC1.
-
ASchematic diagram of the functional regions of YTHDC1 and FIP1L1 and fragment cloning. The prolines in the proline‐rich region (356–406) of FIP1L1 were mutated into alanines.
-
BCo‐IP assays using truncated FIP1L1 proteins revealed that the proline‐rich domain of FIP1L1 plays an important role in interacting with YTHDC1.
-
CMutation of FIP1L1 nearly abrogates the interaction between YTHDC1 and FIP1L1 compared with wild type.
-
D–GCo‐IP assays using different domains of YTHDC1 revealed that the N‐terminus (1–344 aa) and YTH domain (364–507 aa) of YTHDC1 interact with FIP1L1.
Source data are available online for this figure.
