Synthesis of propargylamine derivatives under the optimized conditionsa.
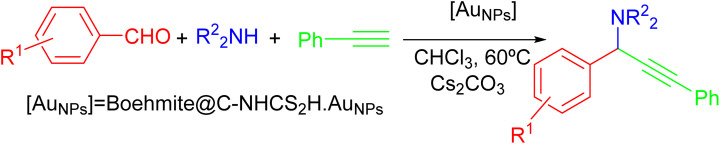
| ||||
|---|---|---|---|---|
| Entry | Time (h) | R22NH | R1 | Yieldb (%) |
| 1 | 12 | Piperidine | H | 96 |
| 2 | 15 | Morpholine | H | 80 |
| 3 | 18 | Piperidine | 4-Me | 75 |
| 4 | 18 | Morpholine | 4-Me | 73 |
| 5 | 16 | Piperidine | 2-Cl | 79 |
| 6 | 18 | Morpholine | 2-Cl | 68 |
| 7 | 18 | Morpholine | 4-OMe | 85 |
| 8 | 16 | Piperidine | 2-Me | 85 |
| 9 | 18 | Morpholine | 2-Me | 73 |
Reaction conditions: 1.2 mmol of morpholine, 1 mmol of benzaldehyde, and 1.3 mmol of phenylacetylene.
Isolated yields in 4 mL of CHCl3.
