Extended Data Fig. 5. Cryo-EM structure of amyloid fibrils generated from moPrP23–144.
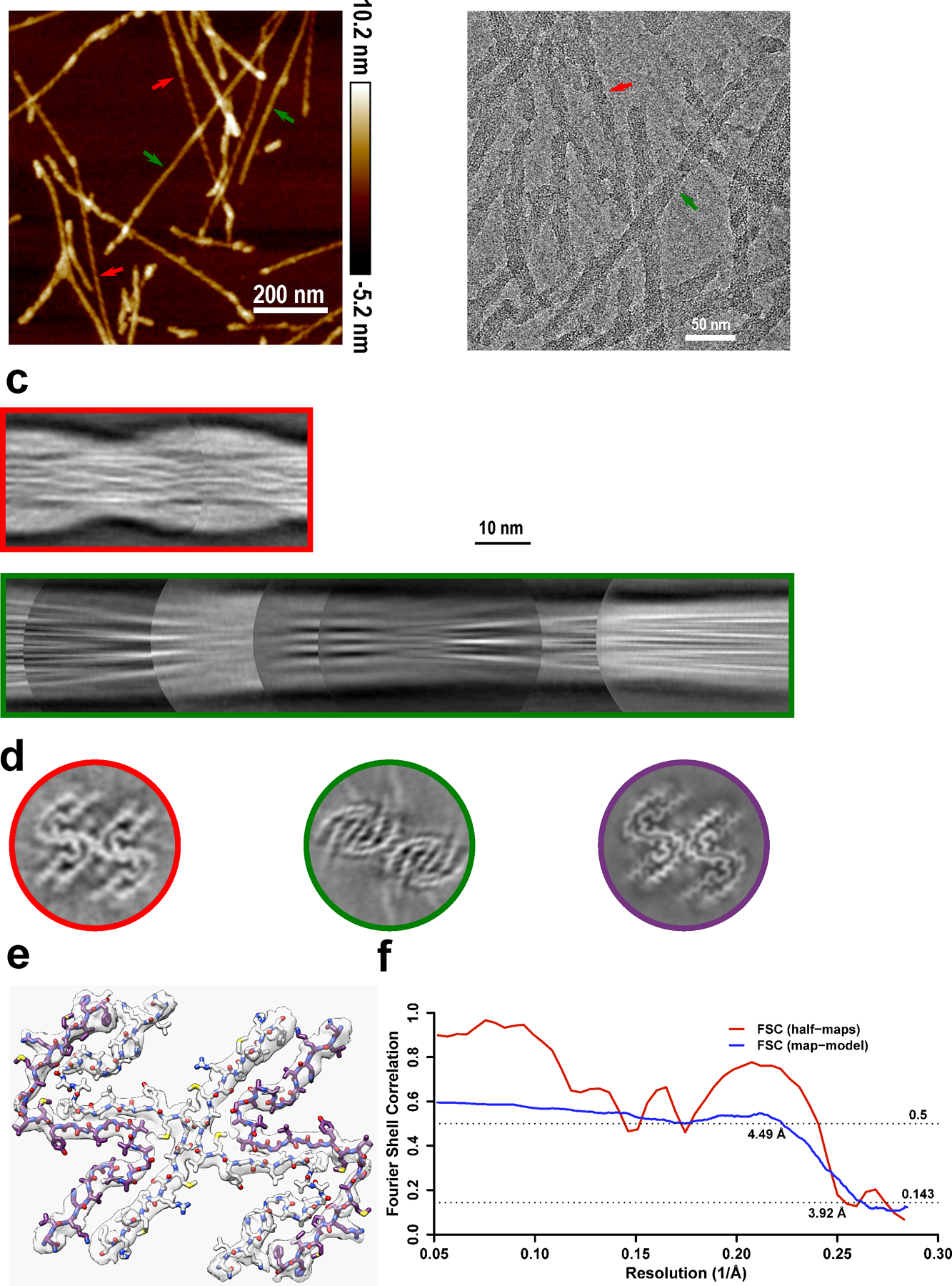
a-b, Two types of polymorphs can be found in AFM images (a) and cryo-EM micrographs (b). Polymorph 1 (red arrows) showed a larger left-handed twist like huPrP23–144 fibrils, and polymorph 2 (green arrows) showed a much smaller right-handed twist. The same fibril morphologies were observed in at least 1,000 cryo-EM images or 20 AFM images for each type of sample. c, Manually assembled half-pitch of both polymorphs from multiple 2D class averages. d, 3D maps of moPrP23–144 and huPrP23–144 fibrils represented by a central slice perpendicular to the fibril axis. Polymorph 1 of moPrP23–144 fibrils (red) showed the same fold as that in huPrP23–144 fibrils (purple). Polymorph 2 of moPrP23–144 fibrils (green) showed a distinctly different fold. e-f, An atomic model built based on the map for polymorph 1 of moPrP23–144 fibrils, with a map resolution of 3.92 Å and model resolution of 4.49 Å. The backbone fold in this model is identical to that in huPrP23–144 fibrils. The structure of polymorph 2 of moPrP23–144 fibrils could not be determined due to poor quality of cryo-EM data for this polymorphic form.
