Abstract
Although vaginal microbiota (VM) may interact with human papillomavirus (HPV) infection and clearance, longitudinal data remain very limited. We aimed to investigate the association between VM at baseline and the clearance of high-risk HPV (HR-HPV) infection within 12 months. Cervical swabs were collected at diagnosis from 85 patients with HR-HPV infection and histologically confirmed cervical lesions, including cervicitis, low-grade squamous intraepithelial lesion and high-grade squamous intraepithelial lesion. Microbiome analysis was performed using 16S rRNA gene sequencing. Among the 73 women included in the analyses, HPV clearance was observed in 58.9% of the patients within 12 months. No significant difference was observed between the HPV-cleared and HPV-uncleared groups regarding age, disease stage, HPV subtype, VM community state types, and VM diversity (α and β). Women with the depletion of enterococcus ASV_62 and enrichment in Lactobacillus iners at baseline were less likely to have HPV clearance at month 12. Further analysis revealed a significant negative association between high abundance of L. iners and HPV clearance in patients who received non-operative treatment (OR = 3.94, p = 0.041), but not in those who received operative treatment (OR = 1.86, p = 0.660). Our findings provide new evidence for the potential role of VM in the persistent HR-HPV infections.
Keywords: vaginal microbiota, high-risk human papillomavirus, HPV clearance, longitudinal study, cervical lesions
Introduction
Persistent infection with high-risk human papillomavirus (HR-HPV) is the major cause of cervical cancer and its precursor lesion (1–3). Although HPV infection is common worldwide, approximately 80% of the HPV infections are transient and cleared spontaneously within 2 years, and the remaining 20% of cases ensue persistent infection and disease progression. However, the exact factors that determine infection and/or disease that will persist, progress, or spontaneously resolve are incompletely understood.
More recently, the upsurge in research on microbiome has shifted attention from known epidemiological risk factors of HPV infection, including parity, tobacco smoking, and oral contraceptive use to mucosal microbiota (4). Accumulating evidence has revealed that mucosal microbiota plays a critical role in maintaining physiological homeostasis (5). Several recent studies have highlighted the significance of the microbiome in the natural history of various viral infections and cancers (6, 7). The cervicovaginal microbiome is of particular interest in gynecology because it has been well characterized and several specific features have been associated with gynecologic diseases (8–10).
The vaginal microbiota (VM) is commonly categorized into community state types (CSTs), which were first proposed by Ravel et al. (9), and are generally defined as a dominant of a specific Lactobacillus spp. or a polymicrobial state with high diversity. Several cross-sectional studies (11–17) have observed distinct characteristics of VM between healthy controls and patients with HPV infection or squamous intraepithelial lesion (SIL). A link between high abundance of some types of Lactobacillus (L. crispatus, L. jensenii, and L. gasseri) and low HPV prevalence is generally supported (18). Contrary to the beneficial role of these Lactobacillus spp., current evidence with regard to L. iners, which was common in patients with HR-HPV infections and high-grade cervical lesions, remains inconsistent. In addition, high-diversity CSTs and specific anaerobes, such as Sneathia and Gardnerella vaginalis, were also found to be implicated with higher frequency and severity of disease. Nevertheless, the results of previous studies are inconsistent and sometimes contradictory, possibly because of the differences in the genetic background or environmental factors (18, 19). Additionally, it remains unclear whether features of VM influence the clearance or instead promote disease development.
Herein, we conducted a longitudinal study to assess the impact of VM composition at baseline on the clearance of HR-HPV infections at 12 months based on a treatment cohort of 85 Chinese women with a single HR-HPV infection.
Methods
Ethics statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Scientific and Ethical Committee of the Shanghai First Maternity and Infant Hospital affiliated with Tongji University (protocol code: K08-018).
Study design
Patients with histologically diagnosed cervical disease were enrolled between April 2015 and October 2016 at Shanghai First Maternity and Infant Hospital and then routinely followed up every 6 months. Cervical swab specimens at diagnosis were collected from every woman for HPV testing and microbiome analysis using 16S rRNA gene sequencing after inclusion in the cohort. Detailed description of the participants can be obtained from the original publication (20). All the data were collected after obtaining written informed consent from participants.
Inclusion criteria included the following: biopsy-proven clinical lesions of the cervix, including cervicitis, low-grade SIL (LSIL), and high-grade SIL (HSIL); only infected with one HR-HPV subtype (HPV 16/52/58); follow-up data available; and patients with same diagnostic conditions were treated according to a standardized protocol. Briefly, patients with HSIL received the loop electrosurgical excisional procedure (LEEP), and other patients with a lower pathological grade lesion (cervicitis and LSIL) received conservative management rather than immediate excisional treatment. Patients with a known malignancy disease or current pregnancy were excluded from the study.
Follow-up and clinical outcome definition
Based on pathological diagnosis and the treatment undergone, patients were divided into the HPV+/LSIL group and the HSIL group. Cervical swabs and cervical biopsies were collected on each follow-up visit for HPV DNA detection and pathological examination. Clinicopathological and follow-up data, including progress notes, clinical laboratory tests, and drugs and pathological reports were captured from the hospital electronic medical record system. All laboratory examinations and inpatient and outpatient electronic medical records were reviewed.
The HPV-cleared group was defined as the presence of HPV at baseline turned negative at follow-up tests and no further positive HPV test and cytological or histological abnormality reports. The HPV-uncleared group was defined as persistent same-type HPV infection or pathological progress within a 1-year follow-up.
HPV gene type
HPV testing at each follow-up visit was performed using the HPV GenoArray test kit as previously reported (20). The absence of HPV DNA contamination was confirmed by HPV L1 and the internal control of the human a-globin in each reaction.
16S rRNA V4–V5 amplicon sequencing
Microbial genomic DNA was extracted from cervical swab samples, collected from the ectocervix and endocervix of the uterus of every woman by baseline cervical scrapings, using the FastDNA Spin Extraction Kit (MP Biomedicals, Santa Ana, CA, USA). Then, a nested PCR protocol was employed to amplify the 16S hypervariable region V4–V5 using the 16S universal primers: 515F 5’-GTGCCAGCMGCCGCGGTAA-3’ and 907R: 5’-CCGTCAATTCMTTTRAGTTT-3’. The dual-indexed amplicons were pooled according to the manufacturer’s instructions (Illumina, Inc., San Diego, CA, USA) and sequenced on the Illumina MiSeq platform to produce 2 × 300 bp paired-end reads as described previously.
Bioinformatic analysis
The raw demultiplexed sequences were firstly trimmed off primers from the paired-end reads, and a preliminary quality trimming was then conducted with Cutadapt v2.10 with the setting of discard untrimmed sequences, a minimum q-value of 20, and a maximum N base of zero. Thereafter, the processed reads were subjected to quality trimming, denoising, merging, and chimera removal to generate amplicon sequence variants (ASVs) using DADA2 (21). In this step, the paired-end reads were trimmed to keep high-quality reads with a q-value of >20 (maxN = 0, truncQ = 2), and those with more than two or five expected errors [maxEE = c(2,5)] or derived from PhiX (rm.phix = TRUE) were discarded. After DADA2 denoising, the paired-end reads were merged with at least a 12-bp overlap. Chimera checking was conducted on the merged reads, and the recovered ASVs were summarized and used to generate the sequence table for the sequencing run. All ASVs were numbered in order.
Sequencing annotation
RESCRIPt (22) was used to compile trained naïve Bayes classifiers using the SILVA database (v132) (23) and the STIRRUPS vaginal microbiome-specific database (24). As Lactobacillus spp. are essential for further analysis in VM studies, the classification of the ASVs annotated as Lactobacillus was improved by manually BLAST searching them in the National Center for Biotechnology Information database; a maximum of one mismatch was allowed from the alignment. If ASVs could not be annotated at the species level, they were then reannotated as “genus-level ASVs order.”
Diversity analysis
The ASVs table was filtered to exclude sequences annotated as chloroplasts, plastids, or non-bacterial ASVs. Low-abundance ASVs present in only one sample and with a relative abundance lower than 0.01% across all ASVs were also filtered. To reduce the sampling heterogeneity, the ASV table was rarefied to the same reads per sample 100 times using a q2-repeat-rarefy plugin (25) on the QIIME2 platform before conducting diversity analysis. The within-sample (α) diversity was calculated using Chao1 and Shannon indexes based on species richness and species frequencies. The Bray–Curtis distance between samples (β diversity) was used to evaluate differences in species complexity. Both α-diversity indexes and β-diversity distance were calculated using QIIME2 (26).
Clustering into community state types
Hierarchical clustering into CSTs based on VM composition and abundance was conducted according to the methods described by DiGiulio et al. (27). Specifically, the Bray–Curtis distance matrix between all samples was denoised by extracting the most significant principal coordinates analysis (PCoA) eigenvectors. Then, the partitioning around medoids algorithm (pam) was applied to PCoA distances. The number of clusters was determined from the gap statistic. According to this algorithm, VM composition was classified into five groups at the ASV level and two groups at the genus level.
Statistical analysis
Normality tests for continuous data were assessed by Shapiro–Wilk tests. Chi-square tests, Fisher exact tests, t-tests, and Wilcoxon rank-sum tests were used for two-group comparisons as appropriate. For further analysis, numerical variables such as α-diversity indexes were categorized by 75th quartile scores.
PCoA analysis was performed to interrogate the robustness of group-wise clustering. Comparisons of group-wise β diversity (Bray–Curtis distance matrix) were assessed by permutational multivariable ANOVA using the Adonis function in the vegan package (28). Logistic regression models were performed to adjust for known confounders (age, HPV subtype) and calculated adjusted odds ratios (aOR) to evaluate the relationship between CSTs and clinical outcomes. Analyses of differential taxa (species level) abundance of samples according to disease outcome were performed using a negative binomial generalized linear model in the R package DESeq2 (29) with a multifactor design. An adjusted p-value of <0.05 and an estimated fold change of >2 were considered significantly differentially abundant between groups.
P-value was adjusted for multiple tests using the Benjamini and Hochberg method. A p-value of <0.05 was considered statistically significant. Statistical analyses were performed with R statistical programming (R version 3.6.1).
Results
Characteristics of participants in the study cohort and follow-up
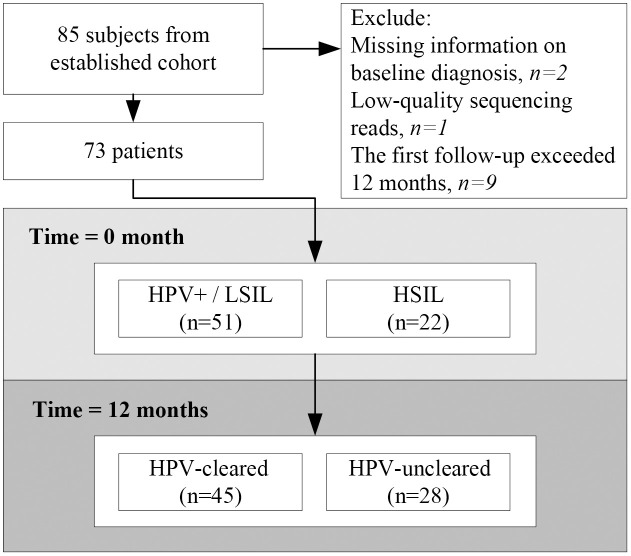
Eighty-five patients with a single HR-HPV infection and histologically confirmed cervical disease were enrolled in this study. Patients with incomplete clinical information (n = 2), low-quality sequence data (n = 1), or time to first follow-up visit over 1 year (n = 9) were excluded. Overall, 73 individuals were included in the analysis ( Figure 1 ). Patient characteristics are detailed in Table 1 . The mean age was 40.1 ± 11.5 years old (median = 36, range: 24–68). The majority of subjects (69.9%) had low-grade cervical lesions. All participants were infected with a single HPV subtype, of whom 37.0% were infected with HPV52, followed by 35.6% with HPV16 and 27.4% with HPV58. Sixteen (21.9%) patients had taken recombinant human interferon α-2b, and five (6.8%) had taken Lactobacillus capsule. No immunomodulatory medication usage was reported. At 12 months, 61.6% (45/73) of patients had cleared HPV infection, defined as the “HPV-cleared” group, and the remaining 38.4% (28/73) were classified as the “HPV-uncleared” group. The clearance rate was 54.9% among HPV+/LSIL patients and 77.3% among HSIL patients. This difference of clearance rate between the two groups was not statistically significant (p = 0.123). No differences in HPV clearance by HPV subtype were noted (HPV16, 65.4%; HPV52, 59.3%; HPV58, 60.0%; p = 0.886). Pairwise comparisons are shown in Supplementary Table S1 .
Figure 1.
Flow chart of the study design. Seventy-three patients with histologically confirmed cervical lesion at baseline entered this study. Follow-up cytology and human papillomavirus (HPV) tests were performed every 6 months to determine whether subjects had cleared HPV infections or not at 12 months.
Table 1.
Clinical characteristics and outcomes of participants in the study.
| Characteristics | HPV-cleared | HPV-uncleared | p-value | Total |
|---|---|---|---|---|
| (N = 45) | (N = 28) | (N = 73) | ||
| Age at diagnosis, years | 0.180 | |||
| Median [range] | 35.0 [24.0–68.0] | 36.5 [27.0–65.0] | 40.1 (11.5) | |
| Diagnosis at baseline | 0.123 | |||
| HPV+/LSIL | 28 (62.2%) | 23 (82.1%) | 51 (69.9%) | |
| HSIL | 17 (37.8%) | 05 (17.9%) | 22 (30.1%) | |
| HPV status | 0.886 | |||
| HPV16 positive | 17 (37.8%) | 09 (32.1%) | 26 (35.6%) | |
| HPV52 positive | 16 (35.6%) | 11 (39.3%) | 27 (37.0%) | |
| HPV58 positive | 12 (26.7%) | 08 (28.6%) | 20 (27.4%) | |
| Drug | ||||
| Recombinant human interferon α-2b | 1.000 | |||
| No | 35 (77.8%) | 22 (78.6%) | 57 (78.1%) | |
| Yes | 10 (22.2%) | 06 (21.4%) | 16 (21.9%) | |
| Lactobacillus capsule | 1.000 | |||
| No | 42 (93.3%) | 26 (92.9%) | 68 (93.2%) | |
| Yes | 3 (6.7%) | 02 (7.1%) | 5 (6.8%) | |
| CSTs | 0.216 | |||
| I | 6 (13.3%) | 04 (14.3%) | 10 (13.7%) | |
| II | 15 (33.3%) | 07 (25.0%) | 22 (30.1%) | |
| III | 6 (13.3%) | 09 (32.1%) | 15 (20.5%) | |
| IV | 10 (22.2%) | 02 (7.1%) | 12 (16.4%) | |
| V | 8 (17.8%) | 06 (21.4%) | 14 (19.2%) | |
| CST_genus | 1.00 | |||
| 1 | 28 (62.2%) | 18 (64.3%) | 46 (63.0%) | |
| 2 | 17 (37.8%) | 10 (35.7%) | 27 (37.0%) | |
| Chao1 | 0.074 | |||
| Median [min, max] | 36.5 [8.00, 134] | 27.3 [6.00, 99.1] | 29.0 [6.00, 134] | |
| Pielou_evenness | 0.863 | |||
| Mean (SD) | 0.387 (0.166) | 0.39 (0.164) | 0.390 (0.164) | |
| Observed_features | 0.073 | |||
| Median [min, max] | 32.0 [8.00, 131] | 26.0 [6.00, 98.0] | ||
| Shannon | 0.664 | |||
| Median [min, max] | 1.56 [0.143, 4.54] | 1.77 [0.327, 3.34] | ||
| Simpson | 0.987 | |||
| Median [Min, max] | 0.55 [0.028, 0.928] | 0.61 [0.0850, 0.822] | ||
SD, standard deviation; HPV, human papillomavirus; CSTs, community state types; LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion. HSIL patients received surgical resection, and LSIL/HPV+ patients underwent non-surgical treatment.
Comparisons between groups were made using chi-square test, t-test, Wilcoxon rank-sum test, and Fisher exact test as appropriate. Age at diagnosis, Chao1, observed features, Shannon, and Simpson were non-normal distribution tested by Shapiro–Wilk test.
Characteristics of baseline vaginal microbiota composition
A total of 2,785,739 high-quality sequences were obtained from 73 samples, with an average of 38,160 reads per sample. Following the removal of rare frequency (singletons and <0.1% total reads), nonbacterial, unclassified, mitochondrial, and chloroplast ASVs, 530 ASVs were finally generated, and then all samples were rarefied to 8,556 reads 100 times to calculate diversity indexes.
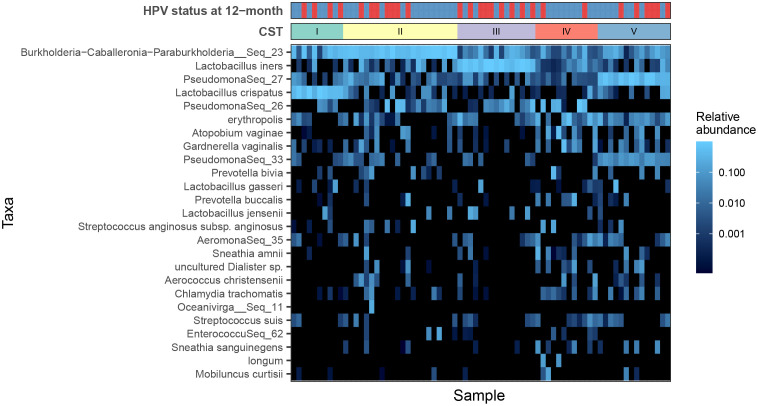
To explore CSTs and reduce dimensionality, a hierarchical clustering analysis was performed based on ASV-level data, and five major groups were identified ( Figure 2 and Figures S1A–E ): CST I (10/73, 13.7%) was classified as L. crispatus dominated, CST III was dominated by L. iners (15/73, 20.5%), and CST IV (12/73, 16.4%) was typified by a highly diverse microbiome rather than containing a major group.
Figure 2.
Heat map of the fractional abundance of the 25 most abundant amplicon sequence variants (ASVs) in the vaginal communities of all subjects. Clustering on the abundance profiles of individual samples using the partitioning around medoids algorithm identifies five community state types. Human papillomavirus (HPV) infection outcomes are indicated by the bar at the top: HPV negative (blue) and HPV positive (red).
A similar genus-level analysis demonstrated that all the samples were mainly separated into two groups, the Lactobacillus-dominated group (CST1, 63.0%) and the non–Lactobacillus-dominated group (CST2, 37.0%) ( Figures S1F, G ). Alpha diversity analysis of the microbiota profile based on Shannon and Chao1 diversity showed that patients with HPV-16 infection or non–Lactobacillus-dominated CST had higher VM diversity ( Tables S2 and S3 ). PCoA plot of Bray–Curtis distances showed a clear separation of microbial composition between different CSTs ( Figure S2 ). Although no significant correlation was observed between VM composition (measured by Bray–Curtis) and clinical variables, including HPV subtype, age, and disease stage ( Tables S4 and S5 ).
Overall vaginal microbiota diversity and HPV clearance
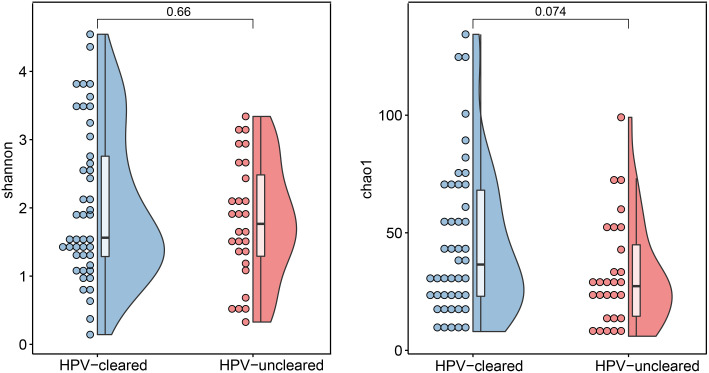
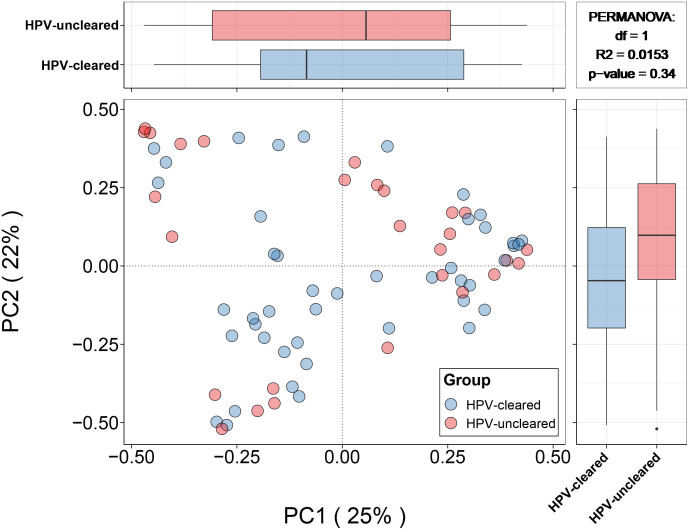
At 12 months, clearance rates appeared to be higher in HSIL patients than in patients with HPV+/LSIL, bordering on significance (77.3 vs. 54.9%, p = 0.123). Both α-diversity and β-diversity analyses showed no significant difference between the HPV-cleared and HPV-uncleared groups ( Figure 3 ). Similarly, Bray–Curtis distance showed no clear separation among samples from different HPV treatment outcome groups ( Figure 4 ). In addition, these data were supported by the fact that no significant difference was found between VM features and HPV outcomes in univariate and multivariate logistic regression analyses ( Table 2 ). Further stratified analysis by HPV subtype and disease stage did not reveal any significant differences between the two groups ( Tables S6 and S7 ).
Figure 3.
Microbial α-diversity analysis based on Shannon and Chao1 index in the HPV-cleared and HPV-uncleared groups. Statistical significance between the groups was tested by Wilcoxon rank sum tests. HPV, human papillomavirus.
Figure 4.
Principal coordinates analysis (for principal coordinates PCo1 and PCo2) plots with Bray–Curtis distance showing the difference in microbial community composition between the HPV-cleared and HPV-uncleared groups. Statistical significance between the groups was tested by permutational multivariable ANOVA. HPV, human papillomavirus.
Table 2.
Association between VM and HPV clearance in a 12-month follow-up.
| Characteristics | Univariable model | Multivariable model 1 | Multivariable model 2 | Multivariable model 3 | Multivariable model 4 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p value | aOR (95% CI) | p value | aOR (95% CI) | p value | aOR (95% CI) | p value | aOR (95% CI) | p value | |
| Age | ||||||||||
| <50 year | Ref. | Ref. | Ref. | Ref. | Ref. | |||||
| ≥50 year | 1.69 (0.96–2.98) | 0.070 | 3.38 (0.89–12.8) | 0.073 | 2.4 (0.71–8.08) | 0.157 | 3.78 (0.97–14.69) | 0.055 | 2.46 (0.73–8.32) | 0.147 |
| HPV type | ||||||||||
| 16 | Ref. | Ref. | Ref. | Ref. | Ref. | |||||
| 52 | 1.3 (0.43–3.96) | 0.646 | 1.2 (0.33–4.38) | 0.782 | 0.93 (0.28–3.09) | 0.901 | 1.31 (0.36–4.82) | 0.683 | 0.97 (0.29–3.29) | 0.963 |
| 58 | 1.26 (0.38–4.20) | 0.708 | 0.99 (0.25–3.94) | 0.987 | 0.78 (0.21–2.96) | 0.720 | 1.09 (0.27–4.43) | 0.908 | 0.81 (0.21–3.07) | 0.755 |
| Diagnosis | ||||||||||
| HPV+/LSIL | Ref. | Ref. | Ref. | Ref. | ||||||
| HSIL | 0.5 (0.22–1.15) | 0.105 | 0.38 (0.1–1.4) | 0.146 | 0.36 (0.11–1.24) | 0.106 | 0.34 (0.09–1.28) | 0.110 | 0.35 (0.1–1.2) | 0.096 |
| Shannon | ||||||||||
| Low | Ref. | Ref. | Ref. | Ref. | Ref. | |||||
| High | 0.75 (0.25–2.30) | 0.614 | 1.14 (0.27–4.76) | 0.858 | 0.84 (0.22–3.16) | 0.794 | 0.87 (0.19–3.95) | 0.858 | 0.7 (0.2–2.49) | 0.577 |
| CSTs | ||||||||||
| I | Ref. | Ref. | Ref. | |||||||
| II | 0.70 (0.15–3.30) | 0.652 | 0.38 (0.07–2.22) | 0.284 | 0.38 (0.06–2.27) | 0.288 | ||||
| III | 2.25 (0.44–11.52) | 0.330 | 1.53 (0.27–8.55) | 0.630 | 1.91 (0.31–11.62) | 0.484 | ||||
| IV | 0.30 (0.04–2.16) | 0.232 | 0.13 (0.01–1.25) | 0.077 | 0.13 (0.01–1.27) | 0.079 | ||||
| V | 1.12 (0.22–5.86) | 0.889 | 0.6 (0.09–4.17) | 0.609 | 0.84 (0.11–6.39) | 0.863 | ||||
| CST_genus | ||||||||||
| I | Ref. | Ref. | Ref. | |||||||
| II | 0.92 (0.34–2.44) | 0.859 | 0.75 (0.22–2.59) | 0.649 | 0.73 (0.24–2.28) | 0.594 | ||||
| Recombinant human interferon α-2b | ||||||||||
| No | Ref. | Ref. | Ref. | |||||||
| Yes | 0.95 (0.3–3) | 0.936 | 2.47 (0.57–10.78) | 0.229 | 1.38 (0.39–4.91) | 0.615 | ||||
| Lactobacillus capsule | ||||||||||
| No | Ref. | |||||||||
| Yes | 1.08 (0.17–6.88) | 0.938 | ||||||||
VM, vaginal microbiota; HPV, human papillomavirus; LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion; CSTs, community state types; aOR, adjusted odds ratios. HSIL patients received surgical resection, and LSIL/HPV+ patients underwent non-surgical treatment.
Certain bacterial species showed correlation to human papillomavirus treatment outcome
Analysis of bacterial taxonomic categories of the microbiome associated with HPV-uncleared versus HPV-cleared was performed using DESeq2, which built a generalized linear model to validate the abundance of each taxon with adjustments for disease stage, age group, and HPV subtype. DESeq2 results showed that abundances of the two species (one specie enriched and one specie depleted in HPV-cleared group) presented a significant difference between two groups. The abundance of Enterococcus:ASV_62 (|log-fold change| = 8.84, q < 0.001) was significantly more prevalent among HPV-cleared patients. Interestingly, patients with higher abundance of L. iners at baseline were more likely to fail to clear HPV infection at 12 months (|log-fold change| = 4.16, q < 0.001).
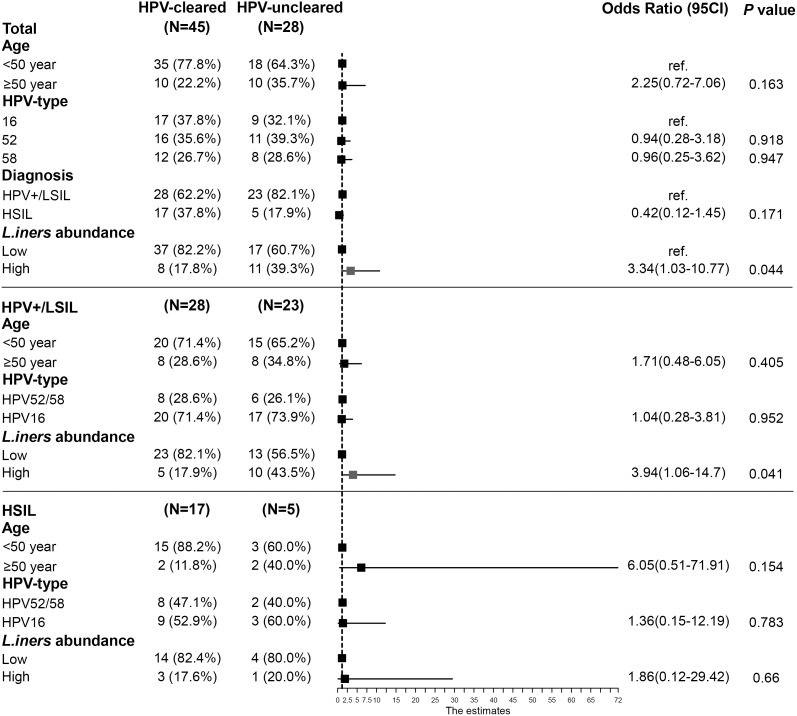
To assess the robustness of the negative correlation between L. iners abundance and HPV clearance, a multivariable logistic regression model and stratified analyses were performed to assess the effect of L. iners abundance on HPV outcome status. The multivariable analysis revealed a significant risk effect of higher L. iners abundance (aOR = 3.34, 95% CI: 1.03–10.77) on HPV clearance ( Figure 5 ). Further stratification logistic analysis showed that this association was only observed in patients with HPV+/LSIL (aOR = 3.94, 95% CI: 1.06–14.70), but not in those with HSIL (aOR = 1.86, 95% CI: 0.12–29.42) ( Figure 5 ).
Figure 5.
Association between L. iners abundance and HPV clearance in a 12-month follow-up. Forest plots were shown using data from multivariable logistic regression in total and according to disease status. HSIL patients received surgical resection, and LSIL/HPV+ patients underwent a surgical treatment. The abundance of L. iners was categorized by 75th quartile scores. HPV, human papillomavirus; LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion.
Discussion
To our knowledge, this longitudinal study reported for the first time that the relative abundance of specific cervicovaginal bacteria, L. iners, rather than the overall diversity of VM, negatively associated with the clearance of HR-HPV at month 12 after diagnosis among patients who received non-operative treatment. Our findings provide new evidence that VM may influence the clearance of HR-HPV infections and suggested a new potential therapy target that merits further investigation.
After adjustment for potential confounders, we observed the association of the enrichment of L. iners and depletion of Enterococcus ASV_62 with the clearance of HR-HPV. Possibly because of the relatively limited sample size, a non-significant similar trend was seen when comparing to the CST I (L. crispatus dominated) state, that patients with CST III (L. iners dominated) state at baseline were associated with a lower rate of HPV clearance. Previously, the possible link between L. iners and persistent HPV infection has been reported in several cross-sectional studies (11, 17, 30, 31). A recent meta-analysis showed that VM dominated by L. iners, compared with VM dominated by L. crispatus was associated with a two- to threefold higher risk of HR-HPV infection and dysplasia (32). Although Usyk et al. (33) found that L. iners was the most positively associated taxon with clearance at 12 months among young adults form Costa Rica (33). The discrepancies in the findings may be related to different inclusion criteria and race differences across studies. By including patients with a single-type HR-HPV infection, this study reduced possible confounding factors related to interactions within different types of HR-HPV.
Compared to other Lactobacillus spp. frequently identified, such as L. crispatus, which is usually considered as a biomarker of healthy vaginal microenvironment, current studies support an ambiguous role for L. iners in the vaginal niche (34, 35). L. iners has a relatively small genome size compared with other Lactobacillus spp. and has been reported as a dominant species in the transitional type of the VM (CST III) or during menses or episodes of bacterial vaginosis, suggesting that this species is very flexible and has a remarkable ability to adapt to the fluctuating vaginal environment (34). The genome of L. iners also encodes a number of genes, suggesting that it could may be an opportunistic pathogen, of which inerolysin (a potential cholesterol-dependent cytolysin) is well documented (36). Besides, a study examining cytokine profiles in pregnancy women showed a positive association between L. iners and proinflammatory cytokines in vaginal fluid (37). However, the available literature is insufficient to classify L. iners as a beneficial or detrimental bacterium. Because L. iners–dominated CST III is frequently reported as one of the most common CSTs among Asian reproductive-age women, a more detailed approach to explore the causal relationship between L. iners and HPV infection clearance is warranted in future vaginal microbiome studies.
Further stratified analysis according to disease status or treatment revealed that the negative correlation between the abundance of L. iners and HPV clearance was only observed in HPV+/LSIL patients who received non-operative treatment. One possible reason is that, compared with non-surgical management such as anti-inflammatory or antiviral treatment, the impact of resection of the lesion on HPV clearance is immediate (38). Recently, Mitra et al. (39) found that the surgical excision for HSIL and HPV infection did not alter VM composition, which suggested that the virus is not the driver of VB alternations. In this study, women with histologically confirmed HSIL were immediately treated surgically according to clinical guidelines, whereas conservative management with regular follow-up was recommended for HPV+/LSIL patients. Previous studies (40, 41) reported that the HR-HPV clearance can reach 79.2%–97.8% (40) at 12 months after LEEP. In this study, 77.3% of HSIL patients and 54.9% of HPV+/LSIL patients were cleared of HPV infection within 12 months, which is comparable to other similar studies (41–43). It is likely that the effect of surgical treatment can mask the correlation between L. iners and HPV outcome in HSIL patients. These findings provide clues for future research regarding the potential targeting populations regarding the association between VM and HPV persistence.
The study has the following limitations. First, the sample size was relatively limited; thus, the negative findings need to be interpreted with caution because of potential insufficient statistical power. Considering the subtype of HPV could be a confounder factor affecting the association between VM and HPV clearance, this study enrolled patients with one of three most prevalent HR-HPV types (HPV16, HPV52, or HPV58). These were the top three subtypes with the highest HPV infection rates according to our previous study (20) and epidemiological surveys in mainland China (44, 45). Further studies with a larger sample size and more types of HPV infection would allow more detailed analyses by HPV subtypes. Second, as cigarette smoking is rare among Chinese women, we were not able to investigate the effect of smoking as a potential confounder. Reported by the Shanghai municipal center for disease control and prevention, the smoking prevalence among female was 1.03% at 2016 (46). The smoking status is not likely to change the reported associations in this study. Third, a single sampling at baseline cannot establish a definitive causal link between L. iners and clearance of HPV infection. Further prospective human studies with repeated sampling and follow-up time longer than 24 months are needed to better clarify the association between VM and persistent HPV infection.
In conclusion, this study among Chinese women suggested that the relative abundance of L. iners at diagnosis, rather than overall bacterial diversity, was negatively correlated with HPV clearance over 12 months, particularly in patients who received non-operative treatment. Our findings provide new evidence for the potential role of VM in the persistent HR-HPV infections. Further studies are needed to clarify the mechanisms by which L. iners promotes persistent HPV infection or lesion progression.
Data availability statement
The raw sequence data reported in this paper have been deposited in the Genome Sequence Archive (Genomics, Proteomics & Bioinformatics 2021) in National Genomics Data Center (Nucleic Acids Res 2021), China National Center for Bioinformation / Beijing Institute of Genomics, Chinese Academy of Sciences (GSA: HRA003171) that are publicly accessible at https://ngdc.cncb.ac.cn/gsa.
Ethics statement
This study was reviewed and approved by Scientific and Ethical Committee of the Shanghai First Maternity and Infant Hospital affiliated with Tongji University. The patients/participants provided their written informed consent toparticipate in this study.
Author contributions
Conceptualization: WS, HZ, ZL and KW. Methodology: WS and XH. Software: WS and LY. Validation: HZ and XC. Formal analysis: WS and HZ. Investigation, XH, LY and WS. Resources, LY, XH and KW. Data curation: XC and HZ. Writing—original draft preparation: WS, HZ and ZL. Writing—review and editing: WS, HZ, LY, XC, XH, KW and ZL. Visualization: W.S. Supervision: HZ and ZL. Project administration: KW and ZL. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by grants from the Shanghai First Maternity and Infant Hospital Science Project (WS, 2020A20) and Clinical Research Plan of SHDC (ZL, SHDC2022CRS050). The granters played no role in study design, development, data collection, or generation of this manuscript. There is no other financial support.
Acknowledgments
We thank all the participating staff for their involvement with this project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.955150/full#supplementary-material
References
- 1. Franco EL, Schlecht NF, Saslow D. The epidemiology of cervical cancer. Cancer J (2003) 9(5):348–59. doi: 10.1097/00130404-200309000-00004 [DOI] [PubMed] [Google Scholar]
- 2. Castellsagué X. Natural history and epidemiology of hpv infection and cervical cancer. Gynecol Oncol (2008) 110(3 Suppl 2):S4–7. doi: 10.1016/j.ygyno.2008.07.045 [DOI] [PubMed] [Google Scholar]
- 3. Bosch FX, de Sanjosé S. The epidemiology of human papillomavirus infection and cervical cancer. Dis Markers (2007) 23(4):213–27. doi: 10.1155/2007/914823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mitra A, MacIntyre DA, Marchesi JR, Lee YS, Bennett PR, Kyrgiou M. The vaginal microbiota, human papillomavirus infection and cervical intraepithelial neoplasia: What do we know and where are we going next? Microbiome (2016) 4(1):58. doi: 10.1186/s40168-016-0203-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Young VB. The role of the microbiome in human health and disease: An introduction for clinicians. Bmj (2017) 356:j831. doi: 10.1136/bmj.j831 [DOI] [PubMed] [Google Scholar]
- 6. Sepich-Poore GD, Zitvogel L, Straussman R, Hasty J, Wargo JA, Knight R. The microbiome and human cancer. Science (2021) 371(6536): 1–13. doi: 10.1126/science.abc4552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stefan KL, Kim MV, Iwasaki A, Kasper DL. Commensal microbiota modulation of natural resistance to virus infection. Cell (2020) 183(5):1312–24.e10. doi: 10.1016/j.cell.2020.10.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mendling W. Vaginal microbiota. Adv Exp Med Biol (2016) 902:83–93. doi: 10.1007/978-3-319-31248-4_6 [DOI] [PubMed] [Google Scholar]
- 9. Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U.S.A. (2011) 108 Suppl 1:4680–7. doi: 10.1073/pnas.1002611107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen C, Song X, Wei W, Zhong H, Dai J, Lan Z, et al. The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat Commun (2017) 8(1):875. doi: 10.1038/s41467-017-00901-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Piyathilake CJ, Ollberding NJ, Kumar R, Macaluso M, Alvarez RD, Morrow CD. Cervical microbiota associated with higher grade cervical intraepithelial neoplasia in women infected with high-risk human papillomaviruses. Cancer Prev Res (Phila) (2016) 9(5):357–66. doi: 10.1158/1940-6207.CAPR-15-0350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen Y, Qiu X, Wang W, Li D, Wu A, Hong Z, et al. Human papillomavirus infection and cervical intraepithelial neoplasia progression are associated with increased vaginal microbiome diversity in a Chinese cohort. BMC Infect Dis (2020) 20(1):629. doi: 10.1186/s12879-020-05324-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cheng L, Norenhag J, Hu YOO, Brusselaers N, Fransson E, Ahrlund-Richter A, et al. Vaginal microbiota and human papillomavirus infection among young Swedish women. NPJ Biofilms Microbiom (2020) 6(1):39. doi: 10.1038/s41522-020-00146-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chorna N, Romaguera J, Godoy-Vitorino F. Cervicovaginal microbiome and urine metabolome paired analysis reveals niche partitioning of the microbiota in patients with human papilloma virus infections. Metabolites (2020) 10(1): 1–14. doi: 10.3390/metabo10010036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Godoy-Vitorino F, Romaguera J, Zhao C, Vargas-Robles D, Ortiz-Morales G, Vazquez-Sanchez F, et al. Cervicovaginal fungi and bacteria associated with cervical intraepithelial neoplasia and high-risk human papillomavirus infections in a Hispanic population. Front Microbiol (2018) 9:2533. doi: 10.3389/fmicb.2018.02533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Oh HY, Kim BS, Seo SS, Kong JS, Lee JK, Park SY, et al. The association of uterine cervical microbiota with an increased risk for cervical intraepithelial neoplasia in Korea. Clin Microbiol Infect (2015) 21(7):674.e1–9. doi: 10.1016/j.cmi.2015.02.026 [DOI] [PubMed] [Google Scholar]
- 17. Seo SS, Oh HY, Lee JK, Kong JS, Lee DO, Kim MK. Combined effect of diet and cervical microbiome on the risk of cervical intraepithelial neoplasia. Clin Nutr (2016) 35(6):1434–41. doi: 10.1016/j.clnu.2016.03.019 [DOI] [PubMed] [Google Scholar]
- 18. Mortaki D, Gkegkes ID, Psomiadou V, Blontzos N, Prodromidou A, Lefkopoulos F, et al. Vaginal microbiota and human papillomavirus: A systematic review. J Turk Ger Gynecol Assoc (2019) 21(3), 193. doi: 10.4274/jtgga.galenos.2019.2019.0051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kyrgiou M, Mitra A, Moscicki AB. Does the vaginal microbiota play a role in the development of cervical cancer? Transl Res (2017) 179:168–82. doi: 10.1016/j.trsl.2016.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huang X, Li C, Li F, Zhao J, Wan X, Wang K. Cervicovaginal microbiota composition correlates with the acquisition of high-risk human papillomavirus types. Int J Cancer (2018) 143(3):621–34. doi: 10.1002/ijc.31342 [DOI] [PubMed] [Google Scholar]
- 21. Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJ, Holmes SP. Dada2: High-resolution sample inference from illumina amplicon data. Nat Methods (2016) 13(7):581–3. doi: 10.1038/nmeth.3869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Robeson MS, O’Rourke DR, Kaehler BD, Ziemski M, Dillon MR, Foster JT, et al. Rescript: Reproducible sequence taxonomy reference database management for the masses. PLoS computational biology (2020) 17(11): e1009581. doi: 10.1101/2020.10.05.326504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, et al. The Silva ribosomal rna gene database project: Improved data processing and web-based tools. Nucleic Acids Res (2013) 41(Database issue):D590–6. doi: 10.1093/nar/gks1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fettweis JM, Serrano MG, Sheth NU, Mayer CM, Glascock AL, Brooks JP, et al. Species-level classification of the vaginal microbiome. BMC Genomics (2012) 13(8):S17. doi: 10.1186/1471-2164-13-S8-S17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xia Y. Q2-Repeat-Rarefy: Qiime2 plugin for generating the average rarefied table for library size normalization using repeated rarefaction: GitHub repository (2021). Available at: https://github.com/yxia0125/q2-repeat-rarefy.
- 26. Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet C, Al-Ghalith GA, et al. Qiime 2: Reproducible, interactive, scalable, and extensible microbiome data science. PeerJ Preprints (2018) 37(8): 2167–9843. doi: 10.1038/s41587-019-0209-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. DiGiulio DB, Callahan BJ, McMurdie PJ, Costello EK, Lyell DJ, Robaczewska A, et al. Temporal and spatial variation of the human microbiota during pregnancy. Proc Natl Acad Sci U.S.A. (2015) 112(35):11060–5. doi: 10.1073/pnas.1502875112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Oksanen J, B FG, Friendly M, Kindt R, Legendre P, McGlinn D, et al. Vegan: Community ecology package. r package version 2 (2020). Available at: https://CRAN.R-project.org/package=vegan.
- 29. Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for rna-seq data with Deseq2. Genome Biol (2014) 15(12):550. doi: 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mitra A, MacIntyre DA, Lee YS, Smith A, Marchesi JR, Lehne B, et al. Cervical intraepithelial neoplasia disease progression is associated with increased vaginal microbiome diversity. Sci Rep (2015) 5:16865. doi: 10.1038/srep16865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Borgdorff H, Gautam R, Armstrong SD, Xia D, Ndayisaba GF, van Teijlingen NH, et al. Cervicovaginal microbiome dysbiosis is associated with proteome changes related to alterations of the cervicovaginal mucosal barrier. Mucosal Immunol (2016) 9(3):621–33. doi: 10.1038/mi.2015.86 [DOI] [PubMed] [Google Scholar]
- 32. Njoku K, Crosbie EJ. The vaginal microbiota, human papillomavirusand cervical dysplasia a systematic review andnetwork meta-analysis. BJOG (2020) 127(2):181. doi: 10.1111/1471-0528.15867 [DOI] [PubMed] [Google Scholar]
- 33. Usyk M, Zolnik CP, Castle PE, Porras C, Herrero R, Gradissimo A, et al. Cervicovaginal microbiome and natural history of hpv in a longitudinal study. PloS Pathog (2020) 16(3):e1008376. doi: 10.1371/journal.ppat.1008376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Petrova MI, Reid G, Vaneechoutte M, Lebeer S. Lactobacillus iners: Friend or foe? Trends Microbiol (2017) 25(3):182–91. doi: 10.1016/j.tim.2016.11.007 [DOI] [PubMed] [Google Scholar]
- 35. Zheng N, Guo R, Wang J, Zhou W, Ling Z. Contribution of lactobacillus iners to vaginal health and diseases: A systematic review. Front Cell Infect Microbiol (2021) 11:792787. doi: 10.3389/fcimb.2021.792787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Macklaim JM, Gloor GB, Anukam KC, Cribby S, Reid G. At The crossroads of vaginal health and disease, the genome sequence of lactobacillus iners ab-1. Proc Natl Acad Sci U.S.A. (2011) 108 Suppl 1(Suppl 1):4688–95. doi: 10.1073/pnas.1000086107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fettweis JM, Serrano MG, Brooks JP, Edwards DJ, Girerd PH, Parikh HI, et al. The vaginal microbiome and preterm birth. Nat Med (2019) 25(6):1012–21. doi: 10.1038/s41591-019-0450-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Aerssens A, Claeys P, Garcia A, Sturtewagen Y, Velasquez R, Vanden Broeck D, et al. Natural history and clearance of hpv after treatment of precancerous cervical lesions. Histopathology (2008) 52(3):381–6. doi: 10.1111/j.1365-2559.2007.02956.x [DOI] [PubMed] [Google Scholar]
- 39. Mitra A, MacIntyre DA, Paraskevaidi M, Moscicki AB, Mahajan V, Smith A, et al. The vaginal microbiota and innate immunity after local excisional treatment for cervical intraepithelial neoplasia. Genome Med (2021) 13(1):176. doi: 10.1186/s13073-021-00977-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kim YT, Lee JM, Hur SY, Cho CH, Kim YT, Kim SC, et al. Clearance of human papillomavirus infection after successful conization in patients with cervical intraepithelial neoplasia. Int J Cancer (2010) 126(8):1903–9. doi: 10.1002/ijc.24794 [DOI] [PubMed] [Google Scholar]
- 41. Qin Y, Li Q, Ke X, Zhang Y, Shen X, Wang W, et al. Clearance of hr-hpv within one year after focused ultrasound or loop electrosurgical excision procedure in patients with hsil under 30. Int J Hyperthermia (2022) 39(1):15–21. doi: 10.1080/02656736.2021.2010817 [DOI] [PubMed] [Google Scholar]
- 42. Luckett R, Painter H, Hacker MR, Simon B, Seiphetlheng A, Erlinger A, et al. Persistence and clearance of high-risk human papillomavirus and cervical dysplasia at 1 year in women living with human immunodeficiency virus: A prospective cohort study. BJOG (2021) 128(12):1986–96. doi: 10.1111/1471-0528.16758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fonn S, Bloch B, Mabina M, Carpenter S, Cronje H, Maise C, et al. Prevalence of pre-cancerous lesions and cervical cancer in south Africa–a multicentre study. S Afr Med J (2002) 92(2):148–56. [PubMed] [Google Scholar]
- 44. Zhang J, Cheng K, Wang Z. Prevalence and distribution of human papillomavirus genotypes in cervical intraepithelial neoplasia in China: A meta-analysis. Arch Gynecol Obstet (2020) 302(6):1329–37. doi: 10.1007/s00404-020-05787-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li K, Li Q, Song L, Wang D, Yin R. The distribution and prevalence of human papillomavirus in women in mainland China. Cancer (2019) 125(7):1030–7. doi: 10.1002/cncr.32003 [DOI] [PubMed] [Google Scholar]
- 46. Xiao-xia L, Hai-hong Y, Ping-ping B, Ji-ying X, Qing-hua Y, Xin-jian L, et al. Smoking and secondhand smoke exposure among registered residents in shanghai. J Environ Occup Med (2016) 33(10):925–30. doi: 10.13213/j.cnki.jeom.2016.16526 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw sequence data reported in this paper have been deposited in the Genome Sequence Archive (Genomics, Proteomics & Bioinformatics 2021) in National Genomics Data Center (Nucleic Acids Res 2021), China National Center for Bioinformation / Beijing Institute of Genomics, Chinese Academy of Sciences (GSA: HRA003171) that are publicly accessible at https://ngdc.cncb.ac.cn/gsa.