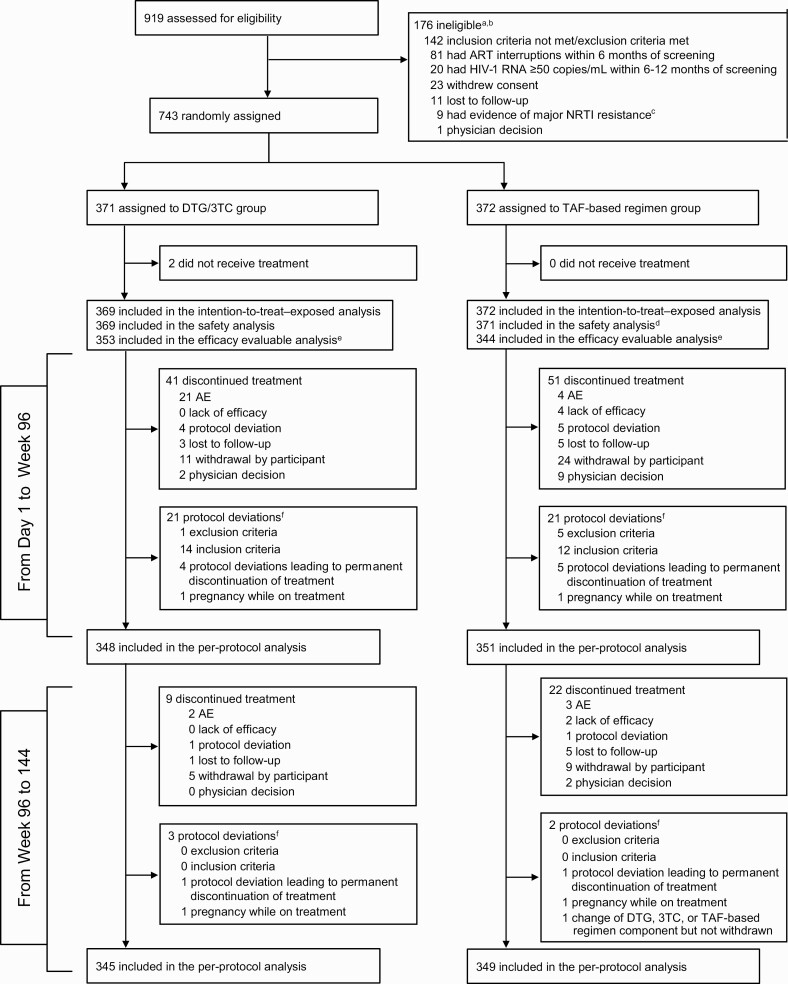
Figure 1.
Trial profile. aParticipants could have multiple reasons for ineligibility. bThe most common reasons for not meeting inclusion criteria or meeting exclusion criteria are listed. All other reasons occurred in <1% of participants. cOf 919 screened participants, 543 (59%) had historical genotypic reports, 9 (2%) of whom were excluded because of major nucleoside reverse-transcriptase inhibitor (NRTI) resistance: 1 had M41L and D67N, 2 had M41L, and the remaining 6 each had a single mutation identified as M184I, K65R, K219E, K219Q, D67N, or L210W (post hoc analysis). dOne participant in the tenofovir alafenamide (TAF)–based regimen group was found to be taking a tenofovir disoproxil fumarate–based regimen at baseline and therefore was excluded from the safety population. eForty-four participants had missing week 96 human immunodeficiency virus type 1 (HIV-1) RNA data owing to the coronavirus disease 2019 pandemic (ie, discontinued or unable to attend week 96 site visit because of the pandemic). fProtocol deviations leading to exclusion from the per-protocol population; participants could have had ≥1 reason. Abbreviations: AEs, adverse events; ART, antiretroviral therapy; DTG/3TC, dolutegravir/lamivudine.