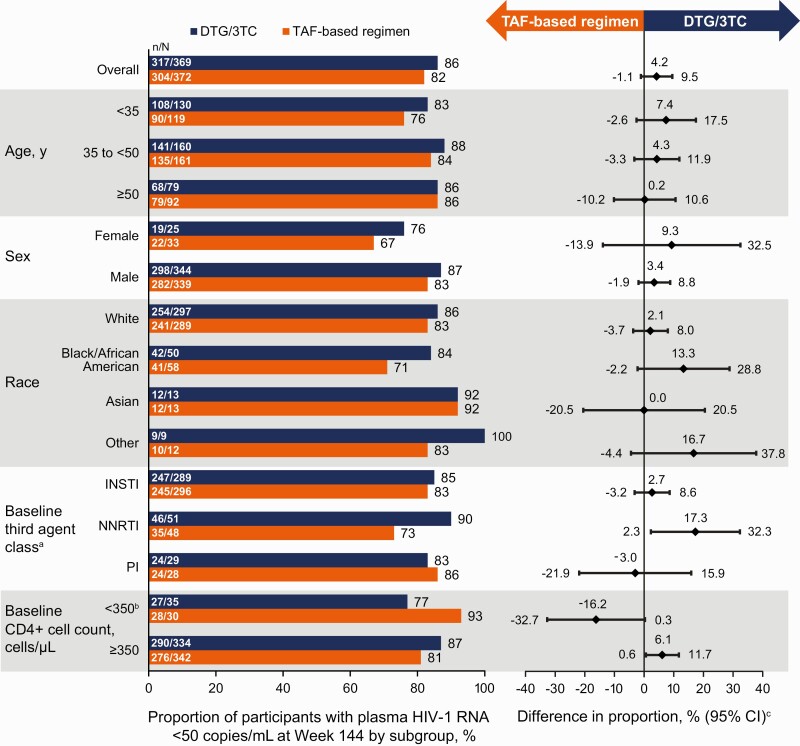
Figure 3.
Proportion of participants with human immunodeficiency virus type 1 (HIV-1) RNA levels <50 copies/mL by subgroup in the intention-to-treat–exposed population by US Food and Drug Administration Snapshot algorithm at week 144. aThe study population was stratified by baseline third agent class (protease inhibitor [PI], integrase strand transfer inhibitor [INSTI], or nonnucleoside reverse-transcriptase inhibitor [NNRTI]). bIn all 8 Snapshot nonresponders receiving dolutegravir/lamivudine (DTG/3TC) with a baseline CD4+ cell count <350/µL, Snapshot nonresponse occurred for nonvirologic reasons. cAdjusted difference for overall population (DTG/3TC − tenofovir alafenamide [TAF]–based regimen) and 95% confidence intervals (CIs) are based on a stratified analysis (adjusting for baseline third agent class) using Cochran-Mantel-Haenszel weights (meeting noninferiority based on −8% margin); unadjusted differences for subgroups represent proportion on DTG/3TC − proportion on TAF-based regimen.