Scheme 3. Scope of Cu(I)-Catalyzed Thio(seleno)glycosylationc.
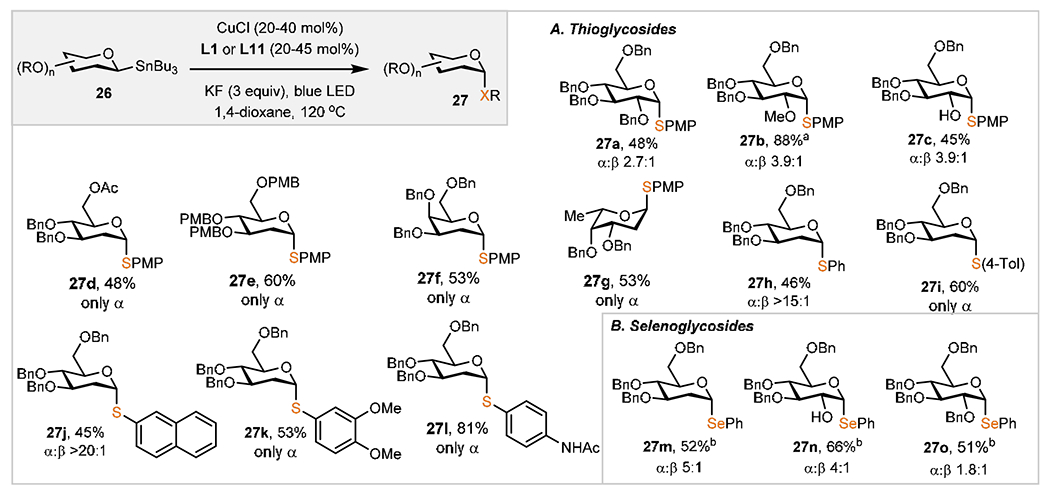
aCuCl (20 mol %), 26 (1.5 equiv), L1 (25 mol %), KF (3 equiv), 1,4-dioxane (2 mL), 130 °C, 96 h. bCuCl (20 mol %), 26 (1.5 equiv), L1 (25 mol %), KF (3 equiv), m-xylene:1,4-dioxane (1:1, 2.00 mL), 130 °C, 96 h. cGeneral reaction conditions: diaryl disulfides or diaryl diselenides (0.100 mmol, 1 equiv), CuCl (20–40 mol %), 26 (1.5 equiv), L11 (25–45 mol %), KF (3 equiv), blue LED (5 W), 1,4-dioxane (2 mL), 120 °C, 96 h; isolated yields. Anomeric selectivities determined by 1H NMR analysis of unpurified reaction mixtures.
