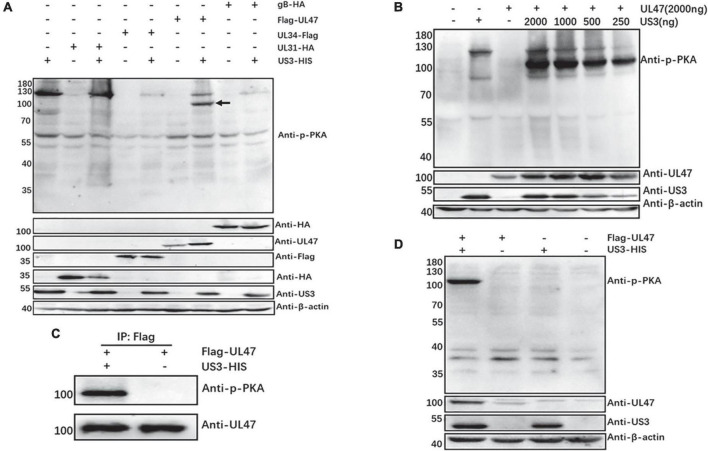
FIGURE 1.
Duck plague virus (DPV) UL47 protein was a phosphorylated substrate of US3 protein. (A) The phosphorylation of UL47 protein was detected in the presence of US3 protein using an anti-phospho-PKA substrate antibody. The expression plasmid gB-HA, UL31-HA, UL34-Flag, or Flag-UL47 was co-transfected with US3-HIS in HEK 293T cells, and their control groups were no expression of US3 protein. At 48 h post-transfection, cells were lysed and analyzed by western blot. The anti-phospho-PKA substrate antibody was used to detect the phosphorylation of gB, UL31, UL34, and UL47 proteins, anti-HA tag antibody was for gB and UL31 protein expression, and anti-Flag tag antibody was for UL34 protein expression. Black arrow marks the phosphorylated UL47 band. (B) The intensity of UL47 phosphorylation depended on US3 protein concentration. The expression plasmid Flag-UL47 was co-transfected with different concentrations of US3-HIS in HEK 293T cells. At 48 h post-transfection, cells were lysed and analyzed by western blot. (C) The enrichment of UL47 protein co-transfected with US3 was detected to be phosphorylated. The plasmid of Flag-UL47 was co-transfected with or without US3-HIS in HEK 293T cells, and then transfected cells were lysed at 48 h post-transfection to perform immunoprecipitation assay using anti-Flag tag antibody and western blot analysis to detect UL47 protein phosphorylation and expression. (D) DPV UL47 protein was still phosphorylated by US3 protein in DEF cells. DEF cells were co-transfected with Flag-UL47 and US3-HIS plasmids and were performed in western blot assays at 48 h post-transfection.