Abstract
Objective: Jatropha mollissima is one of the most valuable medicinal plants used for the treatment of hepatic disorders. It is evident that 500 mg/kg of sodium valproate causes the hepatotoxicity, ototoxicity, gastrotoxicity, bone marrow suppression, and inflammation. This study was designed to explore the medicinal uses of Jatropha mollissima in hepatic disorders. Methods: Hepatotoxicity was induced in Wister albino rats by injecting sodium valproate at the rate of 500 mg/kg once daily for fourteen days. Six male rats, each weighing 220-270 g, were placed into four separate groups for the study. The first group was treated with normal saline. Treatment of the second group was carried out by SVP for four days consecutively together with saline for three weeks. Group three and four were treated with sodium valproate and Jm hydroalcoholic extract applied in the concentrations of the 200 mg/kg and 400 mg/kg for the period of the three weeks. Phytochemical screening and HPLC analysis were conducted to identify the phytochemical nature and polyphenols in extract, respectively. DPPH, SOD, and NO tests were performed to measure the antioxidant activity. Results: With the initial dose of treatments to rats, anatomic, physiological, or histopathologic abnormalities were detected. After three weeks, extract of Jatropha mollissima was used to treat the valproic acid-induced hepatotoxicity (P < 0.05). Conclusion: It was concluded that sodium valproate (SVP) and Jm extract were administered together. The hepatoprotective effects were extraordinarily high, with high concentrations of 400 mg/kg.
Keywords: Jatropha mollissima, hepatotoxicity, reactive oxygen species, sodium valproate
Introduction
Sodium valproate (SVP) is used as a chemical for controlling infections caused by bipolar disorders. In rats, pentylenetetrazol-induced seizures were effectively avoided by sodium valproate [1,2]. As an antiepileptic, SVP is used around the world for the treatment of epilepsy and seizures in children and adults [3]. SVP (sodium valproate) side effects include teratogenicity and liver cirrhosis [4]. SVP causes fatal liver injuries among patients under chemotherapy [5]. Some people have noticed changes in their triglycerides and cholesterol levels because of the negative consequences of SVP [6].
Biochemical reactions occurring through beta acid oxidation inhibition, gluconeogenesis, and urea synthesis are correlated with sodium valproate in the rat hepatocytes [7]. The micro-vesicular ketoacidosis of 500-750 mg/kg of SVP is consistent with a substantial dose of SVP. These effects are induced by depressed protein synthesis through mitochondrial damage, which inhibit fatty acid oxidation and increases the liver mobilization from fat depot sites [8]. Flavonoids are naturally occurring antioxidants and anti-inflammatory compounds in plants. For treating and preventing many diseases, antioxidants serve a crucial role in decreasing oxidative damage to tissues [9]. The hepatoprotective medicament vegetables are observed as an antioxidant characteristic of flavonoids and a propensity to defend the membrane [10,11]. Plants are a natural source of antioxidants and are used to reduce the oxidative stress. Plants contain antioxidants and are used in therapeutic research because of their ability to alleviate oxidative stress [12,13].
Jatropha L. family (Euphorbiaceae) and its subspecies Crotonoideae and Jatropheae are comprised of over 300 species and are widely dispersed throughout the tropical areas of Asia and the United States. The name Jatropha was obtained from the Greek words “jatros” (specialist) and “trophe” (food), which conceivably corresponded with therapeutic qualities of plants from this family [14]. The species Jatropha were used to treat various infectious diseases. Different types of Jatropha species, their medicines, chemical components, and bioactivities have been screened for use as medical plants. Curcas, J elliptica, and mollissima, have been approved for the treatment of infectious disorders [15]. J. mollissima is utilized as antiophyte [16-18] and anti-inflammatory [19] remedies, healing [17], and veterinary vermifuge [16]. It cures kidney diseases and loss of appetite [17]. Jatropha species showed the antioxidant [20], antimicrobial [21], and anthelmintic actions [22].
Some plants have demonstrated SVP-induced hepatotoxicity, but no approved drug exists to restore hepatocyte after SVP damage. There is no evidence to support its use as a liver-protective agent. Jatropha mollissima hepatoprotective properties were evaluated in Wistar albino rats exposed to sodium valproate-induced oxidative stress in this study.
Materials and methods
Chemicals
Sodium valproate, 2,2-diphenyl, 1-picrylhydrazle, formalin, ketamine, and xylazine were purchased from Prix lab Lahore, Pakistan. Ethanol (99.2% pure) was acquired from Lahore Laboratory Chemicals in Pakistan. All chemicals employed in this investigation were of high quality.
Sources and preparation of plant extract
The Jatropha mollissima was procured from the Multan, Pakistan, market. A specialist taxonomist helped authenticate the plant using the voucher number. (R.R. Stewart F.W. Pak.725/16) as a point of reference. A powder form of the Jm plant was obtained by putting the plant material into the blender’s shade for fifteen days. The powdered Jm plant was dissolved to hydroalcoholic solvent for nine days in 4 L colored air-tight containers. The extract of J. mollissima was kept in a refrigerator (-4°C) [23,24].
Animals
Male Wistar albino rats weighing 220-270 g were supplied by the Pharmacology Research Laboratory, Muhammad Institute of Medical and Allied Sciences, Multan. They housed the animals in polycarbonate cages filled with raw dust that was replaced every three days. Under authorization no MIMAS/03-16/34059, experiments were carried out after receiving approval from the Ethical Committee of Muhammad Institute of Medical and Allied Sciences, Multan, Pakistan’s and in accordance to the National Research Council [24,25].
Experimental design
The rats were separated into four groups of six each. Each group consisting of six rats. For the twenty-one days of the experiment, participants in Group 1 were administered a placebo. This sterile saline was administered orally. Group 1 served as the control group. Rats in Group 2 (hepatotoxicity) received SVP in a 500 mg/kg intraperitoneal injection for four consecutive days and normal saline until the twenty-first day of the study. Group 3 rats received SVP at the rate of 500 mg/kg i.p for four consecutive days and Jm 200 mg/kg for three weeks. Group 4 rats received SVP at the rate of 500 mg/kg i.p for four consecutive days and Jm 400 mg/kg for three weeks.
Prior to the experiment, the livers were weighed after they were slaughtered. At zero, seven, and twenty-one days, each experimental rat blood samples were taken and centrifuged at 2300 rpm to extract the serum for the analysis of liver biomarkers [26,27].
Screening of phytoconstituent
The phytoconstituents evaluation was evaluated by using the standardized methodology for the identification of bioactive substances and active chemicals found in the hydroalcoholic extract of Jatropha mollissima [28,29].
HPLC analysis
Following the USP and ICH criteria for phenolic acids and polyphenols, HPLC (High Performance liquid chromatography) analysis was performed [30]. The polyphenols were identified using a 280 nm wavelength. HPLC analysis was performed by using the Ultimate 3000 liquid chromatography system, including a 5 cm flow cell DAD and Chromeleon system management. An amount of 30 mg of dry methanol and a 25 mm mobile phase solvent extract were dissolved separately. Membrane filtering of the sample solution was performed prior to injection into the HPLC apparatus (methanol: 0.5% acetic acid in water: 1.9). Each sample was run for 105 minutes through a High-performance Liquid Chromatography (HPLC) examination utilizing methanol and acetic acid solutions as the mobile solvents. Each standard was recorded and saved in the HPLC spectrum library. The HPLC standard library was used to compare the retention duration and spectrum of unknown substances to determine the identification criteria for Jatropha mollissima chemicals. The phenolic acids and flavonoids in the extracts were measured by placing spikes on the calibration graph against the standard control sample. Three separate data assessments were conducted. The results are shown as a standard error means for each.
Acute toxicity test
An acute toxicity test was performed by following the OECD guidelines. Twenty-four rats were divided into six groups of four rats each. They were deprived of food for 24 hours before receiving doses of 500, 1000, 1500, 2000, 2500, and 3000 mg/kg orally. For fourteen days of taking the J. mollissima, the rats were inspected for jerkiness, tiredness, and death [31].
DPPH assay
Antioxidant activity was measured by DPPH (2,2-Diphenyl-1-picrylhydrazyl) method [32]. The ethanolic extract of leaves was diluted to 1500 and 3000 parts per million (ppm) and 0.5 mL DPPH solution was added to 1 ml of each sample. The absorbance of the solution was measured at 517 nm by using the spectrophotometer. Ascorbic acid was used as a standard in all three investigations. All samples were tested three times and the mean value was calculated.
Biochemical analyses
Various parameters were determined by screening blood samples. A standard kit was used to quantify liver biomarkers levels in blood samples to assess the severity of liver damage.
Estimation of alkaline phosphatase
ALP reagent kit was supplied from Merck to measure the enzyme activity (Darmstadt, Germany). 8 ml of BUF was added into the reaction mixture: Diethanolamine buffer (PH 10.35-0.2) 1.25-0.625 mmol/liter and magnesium chloride 0.625-0.75 mmol/l were mixed together [28,33]. In cuvettes, pipette 20 µl sample and 1000 µl working reagent measured at a temperature of 25°C at a wavelength of 420 nm.
Estimation of alanine transaminase
ALT (Alanine Transaminase) reagent kit was purchased from Merck to measure the alanine transaminase activity (Darmstadt, Germany). A mixture of 8 ml of TRIS buffer (PH 7.4) with 2 ml of substrate i.e., 2-oxoglutarate, NADH, and sodium azide 0.095 percent into an 8 ml of buffer, and mixed completely. After three minutes of mixing the reaction mixture, the absorbance was measured by using the spectrophotometer at wavelength 340 nm.
Estimation of aspartate aminotransferase
AST reagent kit was supplied from Merck to measure the aspartate aminotransferase activity (Darmstadt, Germany). A mixture of 2-oxoglutarate (60 mmol/l), NADH (0.9 mg/l), and sodium azide (0.095 percent) was added to an 8 ml of buffer (TRIS buffer (PH 7.9) 100 mmol/l, L-aspartate (300 mmol/l), and LDH ≥ 1.13 kµ/l MDH ≥ 0.75 kµ/l in combination with sodium azide (0.095 percent). After three minutes of mixing the reaction mixture, the absorbance was measured by using the spectrophotometer at wavelength 334 nm [34].
Estimation of albumin
Albumin was estimated by adding 10 µl of sample into 1000 µl of reagent, i.e., citrate buffer (PH 4.2) 30 mmol/l and Bromo-cresol green 260 mole/l at a wavelength of 546 nm and a temperature of 20-25°C for the measurement of albumin [35,36].
Estimation of total bilirubin
Total bilirubin was measured by following the method by Sazuki et al. [36]. A mixture of 1000 µl of TBR (sulphanilic acid, hydrochloric acid, caffeine (accelerator), sodium benzoate 420 mmol/l, and sodium nitrite) in 1 drop of TNR (T-Nitrate Reagent) and incubated them for 5 minutes. The assay results were compared to the standard curve. 1000 µl of TBR (Total bilirubin reagent) pipette was incubated in cuvettes for 5 minutes [36].
Estimation of direct bilirubin
Total bilirubin was estimated by the method described in previous studies [36]. Sulphanilic acid and hydrochloric acid (HCL) 300 mmol/l was mixed with 1000 µl of DBR and pipetted into 1 drop of DNR (direct nitrite reagent) for the samples and 1000 µl for the sample blank. Within two minutes, pipette 100 µl of sample into cuvettes for both the sample and the sample blank and incubated for 5 minutes at room temperature. The absorbance was measured by a spectrophotometer at wavelength 546 nm.
Histopathological analysis
The livers of rats were dissected under general anesthesia and studied for histopathological purposes. To preserve the liver tissue, it was immersed in a 10% formalin solution before being covered with paraffin. Hematoxylin-eosin was used to stain these samples [28]. SVP’s influence on the cellular architecture of the liver was evaluated using microscopy on hepatic tissue from several groups, alone and in combination with three groups treated with J. mollissima. A compound microscope coupled to a camera LCD was used to acquire micro-images [37,38].
Statistical analysis
A one-way analysis of variance (ANOVA) was used, followed by a Bonferroni’s all-mean post hoc analysis, to conduct the statistical analysis by using Graph Prism Pad. The significance level was set at P < 0.05. The Mean S.E.M. was used to represent the data.
Results
Phytochemical screening and antioxidant activity
The phytochemical analysis revealed the presence of numerous constituents in the hydroalcoholic extract of Jatropha mollissima (Table 1). A hydroalcoholic extract of Jm at a concentration of 1500 g/mL was found to have the highest DPPH level (85.60%). The highest reducing power (79.93%) in various antioxidant experiments; NO (Nitric oxide), H2O2 (Hydrogen peroxide), and SOD (superoxide dismutase) were found to have a 96.43, 90.62, and 76.20% inhibition respectively. As shown in Table 2, antioxidant activity was measured using a wide variety of assays.
Table 1.
Phytoconstituents in extract of the Jatropha mollissima
| Serial No | Test | Ethanolic Extract |
|---|---|---|
| 1 | Flavonoids | Present |
| 2 | Phenols | Present |
| 3 | Tannins | Present |
| 4 | Saponins | Present |
| 5 | Triterpenoids | Absent |
| 6 | Steroids | Absent |
| 7 | Anthrocyanins | Absent |
Table 2.
Findings of various tests on the antioxidant activity of J. mollissima plant extract
| Concentration (μg/ml) | Extract Inhibition | Standard Inhibition | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| DPPH | Reducing power | NO | H2O2 | SOD | DPPH | Reducing power | NO | H2O2 | SOD | |
| 1500 | 85.60 | 79.93 | 96.43 | 90.62 | 76.20 | 98.26 | 79.92 | 96.4 | 95.16 | 93.62 |
| 3000 | 91.20 | 81.53 | 96.56 | 92.65 | 81.34 | 92.28 | 86.39 | 85.25 | 97.28 | 95.82 |
DPPH: 2,2-Diphenyl-1-picrylhydrazyl; SOD: superoxide dismutase; NO: Nitric oxide; H2O2: Hydrogen peroxide.
HPLC analysis
The HPLC analysis showed the presence of different phytoconstituents in various quantities at 280 nm wavelength (Figure 1).
Figure 1.
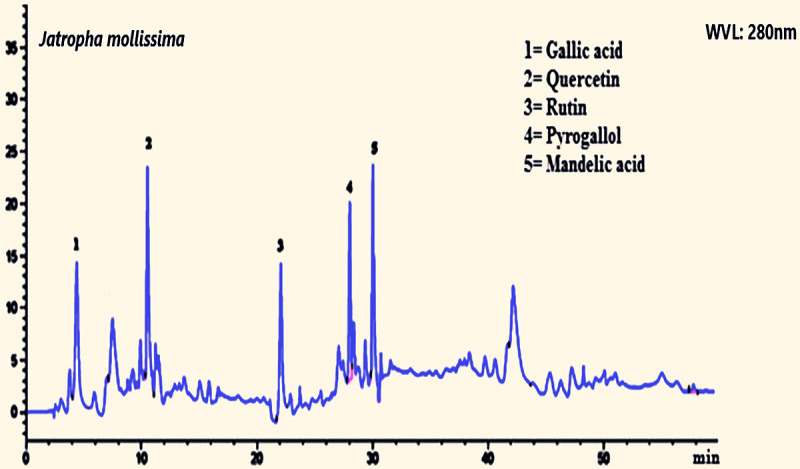
HPLC chromatogram of Jatropha mollissima extract shows rutin, quercetin, mandelic acid, and some others.
Acute toxicity test
There were no convulsions, drowsiness, intolerance, or inflammation in male or female rats at a dose of up to 3000 mg/kg.
Relative organs weight
Rats were administered the dose of 3000 mg/kg concentrations of Jm, and the average relative organ weights were calculated. Relative weight differences between the control and treatment groups were insignificant in terms of kidney, liver, heart, lungs, stomach, and spleen weight (Table 3).
Table 3.
The effect of Jatropha mollissima hydroalcoholic extract on the weight of main organs of the rats
| Treatment | Control Group | Treatment group | |
|---|---|---|---|
|
| |||
| 1500 mg/kg | 3000 mg/kg | ||
| Stomach | 1.4 ± 0.44 | 1.3 ± 0.53 | 1.5 ± 0.56 |
| Heart | 0.86 ± 0.46 | 0.84 ± 0.73 | 0.85 ± 0.76 |
| Liver | 4.93 ± 0.69 | 6.39 ± 0.69 | 6.89 ± 0.72 |
| Kidney | 5.34 ± 0.53 | 4.9 ± 0.71 | 4.7 ± 0.64 |
| Spleen | 1.63 ± 0.49 | 2.13 ± 0.56 | 1.69 ± 0.52 |
| Lung | 1.16 ± 0.73 | 1.96 ± 0.54 | 2.32 ± 0.65 |
Organ/bodyweight (%). Mean ± SEM values (n=5).
Effect of Jm on B.W (body weight) of SVP treated rats
For a period of three weeks, disparities in body weight were observed in all groups on days zero, seven, and twenty-one (Table 4). At day “zero”, there was no significant difference in weight between any of the groups (P > 0.05). It was shown that the weight of the control groups was significantly greater on daysseven and twenty-one. On day seven and twenty-one, a substantial weight gain was seen for those in the SVP-treated group when compared to the control groups weight on day zero. After seven and twenty-one days, the B.W of the SVP + Jma (200 mg/kg) and SVP + Jmb (400 mg/kg) treated animal groups was significantly lower than that of the SVP treated group.
Table 4.
Jatropha mollissima’s effect on B.W of rats treated with SVP
| Groups | Observation (day) | ||
|---|---|---|---|
|
| |||
| 0 | 7th | 21st | |
| B.W (g) | |||
| Control | 235 ± 8.4 | 245 ± 8.3* | 320 ± 7.4* |
| SVP | 248 ± 7.8 | 322 ± 7.0* | 336 ± 7.1* |
| SVP + Jma | 255 ± 8.4 | 294 ± 7.9& | 283 ± 7.8& |
| SVP + Jmb | 289 ± 10.8 | 240 ± 7.7# | 304 ± 10.6# |
Mean ± SEM (n=6), where SVP is sodium valproate (500 mg/kg i.p), SVP + Jma is sodium valproate + Jatropha mollissima extract (200 mg/kg/21 days), SVP + Jmb is sodium valproate + Jatropha mollissima extract (400 mg/kg/21 days).
Indicates P < 0.05 vs normal.
P < 0.05 shows vs SVP.
P < 0.05 Indicates SVP + Jatropha mollissima on corresponding days.
Effect of Jm extracts on ALT and AST of SVP induced rats
Table 5 shows the impact of Jm extracts on AST and ALT levels in SVP-treated rats. Co-administration of SVP and Jm at a rate of 400 mg/kg resulted in a decrease in ALT and AST levels. Sodium valproate elevated AST and ALT levels in rats, but when provided with plant extract at different doses it lowered AST and ALT levels at the doses of 200 and 400 mg/kg. The 400 mg/kg dose rate of SVP + Jma and SVP + Jmb was found to be more efficacious than 200 mg/kg dose rate.
Table 5.
Jatropha mollissima’s effect on AST and ALT of SVP-treated rats
| Groups | Observation (day) | ||
|---|---|---|---|
|
| |||
| 0 | 7th | 21st | |
| AST (u/L) | |||
| Control | 81 ± 6.1 | 75 ± 4.3 | 69 ± 5.1 |
| SVP | 63 ± 5.5 | 180 ± 11* | 171 ± 6.4* |
| SVP + Jma | 71 ± 7.8 | 137 ± 11& | 140 ± 5.6& |
| SVP + Jmb | 69 ± 8.4 | 25 ± 12# | 116 ± 6.0# |
| ALT (u/L) | |||
| Control | 30 ± 4.3 | 41 ± 6.2 | 35 ± 3.5 |
| SVP | 28 ± 3.5 | 98 ± 4.9* | 97 ± 8.9* |
| SVP + Jma | 23 ± 3.6 | 74 ± 6.3& | 70 ± 4.2& |
| SVP + Jmb | 30 ± 3.0 | 60 ± 6.9# | 58 ± 6.0# |
Mean ± SEM (n=6), where SVP is sodium valproate (500 mg/kg i.p), SVP + Jma is sodium valproate + Jatropha mollissima extract (200 mg/kg/21 days), SVP + Jmb is sodium valproate + Jatropha mollissima extract (400 mg/kg/21 days).
Indicates P < 0.05 vs normal.
P < 0.05 shows vs SVP.
P < 0.05 Indicates SVP + Jatropha mollissima on corresponding days.
Effect of Jm on SDB and total bilirubin in SVP-treated rats
Table 6 illustrates that Jm plant extract has a significant effect on serum direct bilirubin and total bilirubin in SVP-induced rats at zero, seven and twenty-one days of dosing. A sodium valproate treatment resulted in a rise in SDB and TBR. Administration of tandemof SVP + Jma and SVP + Jmb at dose rates of 200 and 400 mg/kg reduced these parameters. The 400 mg/kg dose rate of SVP + Jma and SVP + Jmb was found to be more efficacious than the 200 mg/kg dose rate.
Table 6.
Effect of hydroalcoholic extracts of Jatropha mollissima on SDB and TBR of SVP-treated rats
| Groups | Observation (day) | ||
|---|---|---|---|
|
| |||
| 0 | 7th | 21st | |
| SDB (mg/dl) | |||
| Control | 0.11 ± 0.035 | 0.19 ± 0.023 | 0.16 ± 0.041 |
| SVP | 0.15 ± 0.029 | 0.63 ± 0.048* | 0.58 ± 0.060* |
| SVP + Jma | 0.18 ± 0.022 | 0.53 ± 0.051& | 0.45 ± 0.057& |
| SVP + Jmb | 0.14 ± 0.031 | 0.47 ± 0.052# | 0.37 ± 0.049# |
| TBR (mg/dl) | |||
| Control | 0.39 ± 0.050 | 0.43 ± 0.029 | 0.46 ± 0.044 |
| SVP | 0.42 ± 0.041 | 0.94 ± 0.031* | 0.87 ± 0.044* |
| SVP + Jma | 0.43 ± 0.046 | 0.81 ± 0.028& | 0.70 ± 0.038& |
| SVP + Jmb | 0.37 ± 0.053 | 0.75 ± 0.029# | 0.62 ± 0.023# |
Mean ± SEM (n=6), where SVP is sodium valproate (500 mg/kg i.p), SVP + Jma is sodium valproate + Jatropha mollissima extract (200 mg/kg/21 days), SVP + Jmb is sodium valproate + Jatropha mollissima extract (400 mg/kg/21 days).
Indicates P < 0.05 vs normal.
P < 0.05 shows vs SVP.
P < 0.05 Indicates SVP + Jatropha mollissima on corresponding days.
Effect of Jm on albumin and ALP in SVP-treated rats
Table 7 demostrates that ALP and albumin levels in SVP-treated rats as a result of Jm hepatoprotective effects. Albumin and ALP levels were higher in SVP-treated rats. Animals treated with 400 mg/kg of SVP + Jm had lower albumin and ALP levels at zero, seven, and twenty-one days in comparsion with rats having SVP alone.
Table 7.
Hydroalcoholic extracts effects of Jatropha mollissima on ALP and albumin in SVP-treated rats
| Groups | Observation (day) | ||
|---|---|---|---|
|
| |||
| 0 | 7th | 21st | |
| ALP (u/l) | |||
| Control group | 89 ± 6.5 | 107 ± 4.2 | 120 ± 6.8 |
| SVP | 97 ± 9.1 | 225 ± 9.7* | 226 ± 5.1* |
| SVP + Jma | 77 ± 3.7 | 194 ± 5.3& | 172 ± 5.3& |
| SVP + Jmb | 80 ± 3.4 | 179 ± 5.9# | 160 ± 6.8# |
| Albumin (g/dl) | |||
| Control | 4.2 ± 0.12 | 4.3 ± 0.15 | 4.2 ± 0.17 |
| SVP | 4.2 ± 0.12 | 2.6 ± 0.15* | 2.6 ± 0.099* |
| SVP + Jma | 4.3 ± 0.11 | 3.2 ± 0.10& | 3.3 ± 0.14& |
| SVP + Jmb | 4.4 ± 0.11 | 3.4 ± 0.098# | 3.5 ± 0.11# |
Mean ± SEM (n=6), where SVP is sodium valproate (500 mg/kg i.p), SVP + Jma is sodium valproate + Jatropha mollissima extract (200 mg/kg/21 days), SVP + Jmb is sodium valproate + Jatropha mollissima extract (400 mg/kg/21 days).
indicates P < 0.05 vs normal.
P < 0.05 shows vs SVP.
P < 0.05 Indicates SVP + Jatropha mollissima on corresponding days.
Histopathological effects of Jm extract in SVP-treated rats
SVP-treated rats were administered Jm extracts, and the histopathological results are shown in Figure 2. H&E staining was performed to investigate the histopathological effects of Jm plant extract. A uniform structure was found in the normal liver. In the group that received SVP, rancidity was found to be related with substantial hepatic cell damage and liver blockage. There were a few regenerating hepatocytes in the 200 and 400 mg/kg doses of SVP and Jm, including the sinusoidal congestion and scattered inflammatory mononuclear cells, when the two doses were combined.
Figure 2.
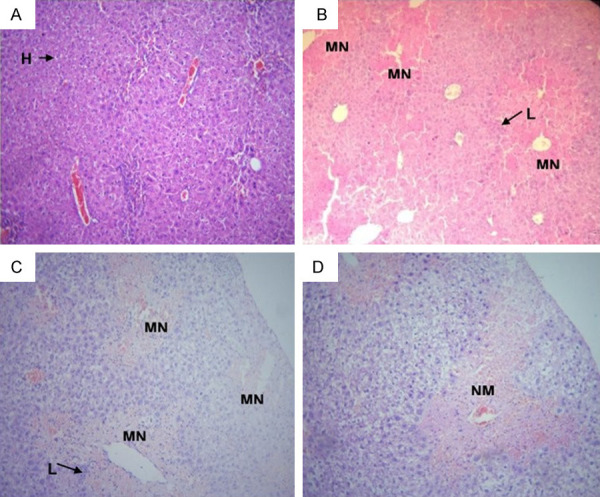
Photomicrograph of liver tissues of (A) control, (B) SVP group, (C) SVP + Jma (200 mg/kg), and (D) SVP + Jmb (400 mg/kg). Showing normal hepatic cells (H), lymphocytic infiltrates (L), multifocal necrosis (M.N.), moderate necrosis (N.M.), mild lymphocytic infiltrates (ML), and mild necrosis (N.L.).
Discussion
The hydroalcoholic extract of Jm was investigated to explore its hepatoprotective properties on SVP-induced hepatotoxicity in albino Wistar rats. Liver injuries are mainly caused by certain environmental and genetic factors [39,40]. Excessive use of some medicines increased the lipid peroxidation and metabolite level in cells and tissues that promotes the production of reactive oxygen species (ROS) [41]. Various medicinal plants possess antioxidants and play significant roles in controlling the oxidative stress faced by cells, aiding in the healing of liver damage.
Previous studies showed that administration of SVP to rats causes a variety of physiological problems, including a decrease in acetyl-CoA concentrations, lipid peroxidation suppression, lipogenesis, gluconeogenicity, and urea production [42,43]. 2,4-Diene Valproyl-CoA, the reactive metabolite of valproic acid, inhibits and down-regulates oxidation enzymes. The oxidative stress and hepatic tissue degeneration produced by SVP were significantly lowered (P > 0.001) by both the 200 and 400 mg/kg dosages of Jm in this investigation (Table 2; Figure 2). The hydroalcoholic extract of Jm was shown to contain rutin, quercetin, and gallic acid, all of which were detected using HPLC (Figure 1). By guarding against nitrosative stress and hepatocyte injury, rutin is a well-known phytoconstituent that shows hepatoprotective effects [44]. Gallic acid showed hepatoprotective effects by altering antioxidant enzyme activities and nonenzymatic antioxidant concentrations [45]. The hepatoprotective effects of quercetin have been documented in previous studies [46]. Phenols found in Jm act as scavengers of free radicals and donors of hydrogen [47]. Secondary plant metabolites are rich in antioxidants and reduce the oxidative stress [48,49].
Our study agreed with the previous studies. It was shown that SVP (sodium valproate) increased the insulin released from beta cells of the pancreas, resulting in an increase in energy conversion and appetite. It was shown that insulin sensitivity and metabolic abnormalities lead to weight gain [50,51]. This research found strong resistance to the SVP-induced rise in organ weight (Table 3).
Our study agreed with the previous studies. In the SVP induced rats, alkaline phosphate, alanine transaminase, and aspartate aminotransferase levels rose because of SVP treatment. The enzyme content in hepatocytes decreased significantly after treatment with Jm. This effect was not observed in the control group (P > 0.001). SVP has been demonstrated to cause liver damage and raise liver enzymes test biomarkers in earlier investigations [52]. Serum albumin levels were restored in both groups treated with Jm, and hepatocellular efficiency was improved, as was reported in earlier studies [53].
Albumin and liver proteins are the best circulating plasma proteins found in serum plasma [54]. Our findings showed that sodium valproate reduced the albumin concentration in sodium valproate treatment rats, resulting in inflammation. Albumin levels significantly declined after Jm injections were delivered to treatment groups. Sodium valproate has been demonstrated to have a deleterious effect on albumin concentrations in previous studies [55-57].
Recent studies showed that serum albumin (total and direct) levels in rats increased after sodium valproate administration, proving the drug to be hazardous. In the Jm plant extract administered group, the amount dropped and was like its typical values. Serum albumin levels followed the onset of sodium valproate toxicity in previous studies [58,59].
SVP has been shown to promote inflammation in vitro and in vivo as indicators to alter the levels of hepatic enzymes and protein biomarkers. The fundamental effects of Jm plant extract on normalizing hepatic enzyme serum levels have been demonstrated. The precise mechanism of action needs to be studied to explore the bioactive compounds. Sodium valproate hepatotoxicity remains a contentious issue [60]. Prior studies on the pathogenesis of hepatocellular necrosis show that comprehensive variables contribute to the development of hepatic necrosis distortion, new blood vessels, and micro-vesicles of hepatic encephalopathy [59-61]. Future studies are needed to explore the mechanism of action of Jm extract in albino Wister rats exposed to different doses of SVP-induced hepatotoxicity.
Conclusions
Our findings showed that ALP, ALAT, ASAT, TBR, and SDB levels were reduced significantly at 200 and 400 mg/kg of Jm extract in albino Wister rats that were exposed to SVP-induced hepatotoxicity by Jm extract. The dose-dependent hepatoprotection of SVP + Jm was observed in this study. Future studies or investigations need to be fully comprehended to explore the mechanism of action of this extract. Different types of Jatropha species, their medicines, chemical components, and bioactivities need to be screened for use as medical plants. Several novel compounds found in Jm need to be studied in greater detail to determine their exact hepatoprotective properties. The discovery of the mechanism underlying its hepatoprotective properties, which requires more molecular study, will open numerous new therapeutic options for the treatment of hepatic illnesses.
Acknowledgements
Hereby we extend our gratitude to the laboratory staff and staff of the animal house of Muhammad Institute of Medical and Allied Sciences, Multan, Pakistan, for their cooperation throughout the study. We are grateful to and Shifa Clinical laboratory Multan to provide research facilities. This study is funded by United Arab Emirates University (UAEU) research grant number 31S414 and UPAR grant (G00003696).
Disclosure of conflict of interest
None.
References
- 1.Burton B. On the propyl derivatives and decomposition products of ethylacetoacetate. Am Chem J. 1882;3:385–395. [Google Scholar]
- 2.Meunier H. Pharmacodynamic properties of N-dipropylacetic acid. Therapie. 1963;18:435–438. [PubMed] [Google Scholar]
- 3.Silva MF, Aires CC, Luis PB, Ruiter JP, IJlst L, Duran M, Wanders RJ, Tavares de Almeida I. Sodium valproate metabolism and its effects on mitochondrial fatty acid oxidation: a review. J Inherit Metab Dis. 2008;31:205–216. doi: 10.1007/s10545-008-0841-x. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt D. Adverse effects and interactions with other drugs. Valproate: Springer; 1999. [Google Scholar]
- 5.Bryant AE 3rd, Dreifuss FE. Sodium valproate hepatic fatalities. III. U.S. experience since 1986. Neurology. 1996;46:465–469. doi: 10.1212/wnl.46.2.465. [DOI] [PubMed] [Google Scholar]
- 6.Wirrell EC. Valproic acid-associated weight gain in older children and teens with epilepsy. Pediatr Neurol. 2003;28:126–129. doi: 10.1016/s0887-8994(02)00505-2. [DOI] [PubMed] [Google Scholar]
- 7.Coude FX, Grimber G, Parvy P, Rabier D, Petit F. Inhibition of ureagenesis by valproate in rat hepatocytes. Role of N-acetylglutamate and acetyl-CoA. Biochem J. 1983;216:233–236. doi: 10.1042/bj2160233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zimmerman HJ, Ishak KG. Valproate-induced hepatic injury: analyses of 23 fatal cases. Hepatology. 1982;2:591–597. doi: 10.1002/hep.1840020513. [DOI] [PubMed] [Google Scholar]
- 9.Mamun-Or-Rashid ANM, Azam MM, Dash BK, Hafiz FB, Sen MK. Ethnomedicobotanical study on Ocimum sanctum L. (Tulsi) - A review. Mintage Journal of Pharmaceutical and Medical Sciences. 2013;2:37–42. [Google Scholar]
- 10.Raza MA, Bekairi OA, Quershi SM. Pathomorphological changes in mouse liver and kidney during prolonged valproate administration. Int J Tissue React. 2000;22:15–21. [PubMed] [Google Scholar]
- 11.Chakraborty S, Majumdar S. Natural products for the treatment of pain: chemistry and pharmacology of salvinorin A, mitragynine, and collybolide. Biochemistry. 2021;60:1381–1400. doi: 10.1021/acs.biochem.0c00629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hazafa A, Rehman KU, Jahan N, Jabeen Z. The role of polyphenol (flavonoids) compounds in the treatment of cancer cells. Nutr Cancer. 2020;72:386–397. doi: 10.1080/01635581.2019.1637006. [DOI] [PubMed] [Google Scholar]
- 13.Singh E, Sharma S, Dwivedi J, Sharma S. Diversified potentials of Ocimum sanctum Linn (Tulsi): an exhaustive survey. J Nat Prod Plant Resour. 2012;2:39–48. [Google Scholar]
- 14.Ahmad N, Fazal H, Abbasi BH, Anwar S, Basir A. DPPH free radical scavenging activity and phenotypic difference in hepatoprotective plant (Silybum marianum L.) Toxicol Ind Health. 2013;29:460–467. doi: 10.1177/0748233712436637. [DOI] [PubMed] [Google Scholar]
- 15.Sabandar CW, Ahmat N, Jaafar FM, Sahidin I. Medicinal property, phytochemistry and pharmacology of several Jatropha species (Euphorbiaceae): a review. Phytochemistry. 2013;85:7–29. doi: 10.1016/j.phytochem.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 16.da Rocha FAG, Dantas LÍS. Atividade antimicrobiana in vitro do látex do aveloz (Euphorbia tirucalli L.), Pinhão bravo (Jatropha mollissima L.) e pinhão roxo (Jatropha gossypiifolia L.) Sobre microrganismos patogênicos. HOLOS. 2009;4:3–11. [Google Scholar]
- 17.Gomes JA, Félix-Silva J, Morais Fernandes J, Geraldo Amaral J, Lopes NP, Tabosa do Egito ES, da Silva-Júnior AA, Maria Zucolotto S, Fernandes-Pedrosa MF. Aqueous leaf extract of Jatropha mollissima (Pohl) bail decreases local effects induced by bothropic venom. Biomed Res Int. 2016;2016:6101742. doi: 10.1155/2016/6101742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ribeiro AR, Andrade FDD, Medeiros MDCD, Camboim ADS, Pereira Júnior FA, Athayde AC, Rodrigues OG, Silva WW. Estudo da atividade anti-helmíntica do extrato etanólico de Jatropha mollissima (Pohl) Baill.(Euphorbiaceae) sob Haemonchus contortus em ovinos no semiárido paraibano. Pesqui Vet Bras. 2014;34:1051–1055. [Google Scholar]
- 19.da Silva Araujo JR, da Silva Costa MW, Oliveira WB, Cavalcante RR, de Almeida PM, Martins FA. Larvicidal, cytotoxic and genotoxic effects of aqueous leaf extract of Jatropha mollissima (Pohl) baill. Acta Scientiarum Biological Sciences. 2018;40:1–7. [Google Scholar]
- 20.de Queiroz Neto RF, de Araújo Júnior HN, Freitas CIA, Costa KMDFM, Abrantes MR, de Almeida JGL, Torres TM, Moura GHF, Batista JS. The Jatropha mollissima (Pohl) baill: chemical and pharmacological activities of the latex and its extracts. Semina: Ciências Agrárias. 2019;40:2613–2624. [Google Scholar]
- 21.Prakash P, Gupta N. Therapeutic uses of Ocimum sanctum Linn (Tulsi) with a note on eugenol and its pharmacological actions: a short review. Indian J Physiol Pharmacol. 2005;49:125–31. [PubMed] [Google Scholar]
- 22.Irshad M, Subhani MA, Ali S, Hussain A. Biological importance of essential oils. Essential Oils-Oils of Nature. 2020 [Google Scholar]
- 23.Singh B, Koley T, Maurya A, Singh P, Singh B. Phytochemical and antioxidative potential of orange, red, yellow, rainbow and black coloured tropical carrots (Daucus carota subsp. sativus Schubl. & Martens) Physiol Mol Biol Plants. 2018;24:899–907. doi: 10.1007/s12298-018-0574-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Institute of Health. Guide for the Care and Use of Laboratory Animals. No. 85-23. Bethesda, MD, USA: NIH Publication; 1985. [Google Scholar]
- 25.Chung IM, Rajakumar G, Lee JH, Kim SH, Thiruvengadam M. Ethnopharmacological uses, phytochemistry, biological activities, and biotechnological applications of Eclipta prostrata. Appl Microbiol Biotechnol. 2017;101:5247–5257. doi: 10.1007/s00253-017-8363-9. [DOI] [PubMed] [Google Scholar]
- 26.Vitins AP, Kienhuis AS, Speksnijder EN, Roodbergen M, Luijten M, van der Ven LT. Mechanisms of amiodarone and sodium valproate induced liver steatosis in mouse in vivo act as a template for other hepatotoxicity models. Arch Toxicol. 2014;88:1573–1588. doi: 10.1007/s00204-014-1211-0. [DOI] [PubMed] [Google Scholar]
- 27.Wang R, Hassan W, Jabeen Q, Ahmed H, Iqbal O. Citrus aurantium ameliorates cisplatin-induced nephrotoxicity. Biomed Res Int. 2019;2019:3960908. doi: 10.1155/2019/3960908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Omer Iqbal M, Bashir Yahya E, Andleeb S, Masood Ahmed M, Umar Javaid M, Shakeel W, Iqbal I. “In vivo assessment of reversing Cisplatin-Induced nephrotoxicity using Jatropha mollissima crude extract and its potential cytotoxicity”. Saudi J Biol Sci. 2021;28:7373–7378. doi: 10.1016/j.sjbs.2021.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trease GE, Evans WC. Trease and Evans’ Pharmacognosy. 16th ed. Edinburgh, Australia: Saunders/Elsevier; 2009. [Google Scholar]
- 30.Seal T, Pillai B, Chaudhuri K. Effect of solvent extraction system on the antioxidant activity of some selected wild leafy vegetables of Meghalaya state in India. Int J Pharm Sci Res. 2013;4:1046–51. [Google Scholar]
- 31.OECD. OECD Guideline for Testing of Chemicals. vol. 420. Paris, France: Organization for Economic Cooperation and Development; 1992. [Google Scholar]
- 32.Mensor LL, Menezes FS, Leitão GG, Reis AS, dos Santos TC, Coube CS, Leitão SG. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother Res. 2001;15:127–130. doi: 10.1002/ptr.687. [DOI] [PubMed] [Google Scholar]
- 33.Maqsood F, Ibrahim T, Farooqi AA, Ahmad MS. Polygonum amplexicaule extract: an effective herbal cure to CCl4 induced liver damage in vivo. J Rare Disord Diagn Ther. 2017;3:4. [Google Scholar]
- 34.Oriakhi K, Patrick O, Ikechi U, Eze G. Hepatoprotective potentials of methanol extract of T. conophorum seeds of carbon tetrachloride induced liver damage in Wistar rats. Clin Phytosci. 2018;4:25. [Google Scholar]
- 35.Parmar SR, Vashrambhai PH, Kalia K. Hepatoprotective activity of some plants extract against paracetamol induced hepatotoxicity in rats. J Herb Med Toxicol. 2010;4:101–6. [Google Scholar]
- 36.Suzuki Y, Sakagishi Y. Determination of serum bilirubin by the Diazo method using the diazotized 3-nitroaniline reacting readily with the photoproducts of bilirubin. Jpn J Clin Chem. 1994;23:158–63. [Google Scholar]
- 37.Gowda S, Desai PB, Kulkarni SS, Hull VV, Math AA, Vernekar SN. Markers of renal function tests. N Am J Med Sci. 2010;2:170. [PMC free article] [PubMed] [Google Scholar]
- 38.Jen JF, Hsiao SL, Liu KH. Simultaneous determination of uric acid and creatinine in urine by an eco-friendly solvent-free high performance liquid chromatographic method. Talanta. 2002;58:711–7. doi: 10.1016/s0039-9140(02)00377-6. [DOI] [PubMed] [Google Scholar]
- 39.Deschamps D, DeBeco V, Fisch C, Fromenty B, Guillouzo A, Pessayre D. Inhibition by perhexiline of oxidative phosphorylation and the β-oxidation of fatty acids: possible role in pseudoalcoholic liver lesions. Hepatology. 1994;19:948–61. [PubMed] [Google Scholar]
- 40.Kassahun KE, Abbott FR. In vivo formation of the thiol conjugates of reactive metabolites of 4-ene SVP and its analog 4-pentenoic acid. Drug Metab Dispos. 1993;21:1098–106. [PubMed] [Google Scholar]
- 41.Cengiz M, Yüksel A, Seven M. The effects of carbamazepine and sodium valproate on the erythrocyte glutathione, glutathione peroxidase, superoxide dismutase and serum lipid peroxidation in epileptic children. Pharmacol Res. 2000;41:423–5. doi: 10.1006/phrs.1999.0603. [DOI] [PubMed] [Google Scholar]
- 42.Pippenger CE. Free radical scavenging enzyme activity profiles in risk assessment of idiosyncratic drug reactions. Probable mechanism for valproate-induced acute pancreatitis and hepatotoxicity. Idiosyncratic reactions to valproate: clinical risk patterns and mechanisms of toxicity. 1991:75–88. [Google Scholar]
- 43.Jurima-Romet M, Abbott FS, Tang W, Huang HS. Cytotoxicity of unsaturated metabolites of sodium valproate and protection by vitamins C and E in glutathione-depleted rat hepatocytes. Toxicology. 1996;112:69–85. doi: 10.1016/0300-483x(96)03352-5. [DOI] [PubMed] [Google Scholar]
- 44.Domitrović R, Jakovac H, Marchesi VV, Vladimir-Knežević S, Cvijanović O, Tadić Ž, Romić Ž, Rahelić D. Differential hepatoprotective mechanisms of rutin and quercetin in CCl 4-intoxicated BALB/cN mice. Acta Pharmacol Sin. 2012;33:1260–1270. doi: 10.1038/aps.2012.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goudarzi M, Kalantar M, Kalantar H. The hepatoprotective effect of gallic acid on mercuric chloride-induced liver damage in rats, Jundishapur. J Nat Pharm Prod. 2017;12:e12345. [Google Scholar]
- 46.Ansar S, Siddiqi NJ, Zargar S, Ganaie MA, Abudawood M. Hepatoprotective effect of quercetin supplementation against acrylamide-induced DNA damage in wistar rats. BMC Complement Altern Med. 2016;16:327. doi: 10.1186/s12906-016-1322-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kpemissi M, Metowogo K, Melila M, Veerapur VP, Negru M, Taulescu M, Potârniche AV, Suhas DS, Puneeth TA, Vijayakumar S. Acute and subchronic oral toxicity assessments of combretum micranthum (combretaceae) in Wistar rats. Toxicol Rep. 2020;7:162–168. doi: 10.1016/j.toxrep.2020.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Godhwani S, Godhwani JL, Was DS. Ocimum sanctum-a preliminary study evaluating its immunoregulatory profile in albino rats. J Ethnopharmacol. 1988;24:193–198. doi: 10.1016/0378-8741(88)90151-1. [DOI] [PubMed] [Google Scholar]
- 49.Chattopadhyay RR. Hypoglycemic effect of Ocimum sanctum leaf extract in normal and streptozotocin diabetic rats. Indian J Exp Biol. 1993;31:891–893. [PubMed] [Google Scholar]
- 50.Yacout GA, Elguindy NM, El Azab EF. Hepatoprotective effect of basil (Ocimum basilicum L.) on CCl4-induced liver fibrosis in rats. Afr J Biotechnol. 2012;11:15702–15711. [Google Scholar]
- 51.Juntachote T, Berghofer E. Antioxidative properties and stability of ethanolic extracts of holy basil and galangal. Food Chem. 2005;92:193–202. [Google Scholar]
- 52.Caragay AB. Cancer-preventive foods and ingredients. Food Technology (Chicago) 1992;46:65–68. [Google Scholar]
- 53.Gang DR, Wang J, Dudareva N, Nam KH, Simon JE, Lewinsohn E, Pichersky E. An investigation of the storage and biosynthesis of phenylpropenes in sweet basil. Plant Physiol. 2001;125:539–555. doi: 10.1104/pp.125.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Devi PU. Radioprotective, anticarcinogenic and antioxidant properties of the Indian holy basil, Ocimum sanctum (Tulasi) Indian J Exp Biol. 2001;39:185–90. [PubMed] [Google Scholar]
- 55.Geetha RK, Vasudevan DM. Inhibition of lipid peroxidation by botanical extracts of Ocimum sanctum: in vivo and in vitro studies. Life Sci. 2004;76:21–28. doi: 10.1016/j.lfs.2004.05.036. [DOI] [PubMed] [Google Scholar]
- 56.Chengappa KN, Chalasani L, Brar JS, Parepally H, Houck P, Levine J. Changes in body weight and body mass index among psychiatric patients receiving lithium, valproate, or topiramate: an open-label, nonrandomized chart review. Clin Ther. 2002;24:1576–1584. doi: 10.1016/s0149-2918(02)80061-3. [DOI] [PubMed] [Google Scholar]
- 57.Luef GJ, Lechleitner M, Bauer G, Trinka E, Hengster P. Sodium valproate modulates islet cell insulin secretion: a possible mechanism of weight gain in epilepsy patients. Epilepsy Res. 2003;55:53–58. doi: 10.1016/s0920-1211(03)00091-3. [DOI] [PubMed] [Google Scholar]
- 58.Kochhar A, Sharma N, Sachdeva R. Effect of supplementation of Tulsi (ocimum sanctum) and Neem (azadirachta indica) leaf powder on diabetic symptoms, anthropometric parameters and blood pressure of non insulin dependent male diabetics. Studies on Ethno-Medicine. 2009;3:5–9. [Google Scholar]
- 59.Mandana SN, Fatemeh N, Hossein N, Azam M, Saleh A. Protective effect of ghrelin on sodium valproate-induced liver injury in rat. Journal of Stress Physiology & Biochemistry. 2013;9:96–105. [Google Scholar]
- 60.Akilavalli N, Radhika J, Brindha P. Hepatoprotective activity of Ocimum sanctum Linn. against lead induced toxicity in albino rats. Asian J Pharm Clin Res. 2011;4:84–87. [Google Scholar]
- 61.Roche M, Rondeau P, Singh NR, Tarnus E, Bourdon E. The antioxidant properties of serum albumin. FEBS Lett. 2008;582:1783–1787. doi: 10.1016/j.febslet.2008.04.057. [DOI] [PubMed] [Google Scholar]


