ABSRACT
The cerebellum has a simple cytoarchitecture consisting of a folded cortex with three cell layers that surrounds a nuclear structure housing the output neurons. The excitatory neurons are generated from a unique progenitor zone, the rhombic lip, whereas the inhibitory neurons and astrocytes are generated from the ventricular zone. The growth phase of the cerebellum is driven by lineage-restricted progenitor populations derived from each zone. Research during the past decade has uncovered the importance of cell-to-cell communication between the lineages through largely unknown signaling mechanisms for regulating the scaling of cell numbers and cell plasticity during mouse development and following injury in the neonatal (P0-P14) cerebellum. This Review focuses on how the interplay between cell types is key to morphogenesis, production of robust neural circuits and replenishment of cells after injury, and ends with a discussion of the implications of the greater complexity of the human cerebellar progenitor zones for development and disease.
Keywords: Purkinje cells, Granule cells, Cerebellar nuclei, Cell plasticity, Injury
Summary: This Review gives an overview of cerebellum development and morphogenesis, which is orchestrated by the coordinated action of cells from various lineages, some of which exhibit plasticity upon injury.
Introduction
The mammalian brain is the most complex organ in the body. It has a vast number of functionally distinct cell types and a complex morphology. Importantly, it is made up of dozens of regions with unique cytoarchitectures that are organized on a local scale for specialized circuit functions but also connect with other regions of the brain to enable the execution of higher-order behaviors through the combined actions of many discrete circuits (Fig. 1A,B). Most brain regions either have a layered (laminar) cytoarchitecture, where different neuron types form distinct layers (e.g. cerebral cortex), or a nuclear structure, where several neuron types group together in a sphere-like structure (e.g. ventral forebrain and midbrain). Layered structures enable greater expansion of the number of cells than nuclear structures through folding of the layer into a compact region (Rakic and Sidman, 1970). The cerebellum, derived from the evolutionarily oldest part of the brain (hindbrain) is an excellent example of how cells within and between brain regions connect. It receives inputs (via axons) from neurons situated throughout the brain and spinal cord and in turn the cerebellum projects back to most regions of the brain and spinal cord (Fig. 1B) (Caligiore et al., 2017). Furthermore, the cerebellum has several subareas that are interconnected. The cerebellum is best known for controlling motor behaviors and balance, but because it connects directly or indirectly through an intermediate neuron with all regions of the nervous system it likely influences all behaviors (Hatten, 2020; Kebschull et al., 2020; Marek et al., 2018; Pisano et al., 2021; Schmahmann, 2019). Indeed, damage to the cerebellum around birth is the second highest risk factor for autism spectrum disorders (Wang et al., 2014). Furthermore, the cerebellum contains 80% of the neurons in the human brain (Azevedo et al., 2009), although it is smaller than the cerebral cortex because the granule cells (GCs) are not myelinated by oligodendrocytes (Leto et al., 2016). Thus, principles of proper cerebellar circuit formation likely have implications for development throughout the brain.
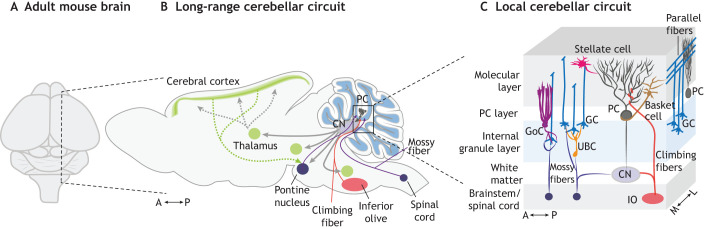
Fig. 1.
Local and long-range cerebellar circuits. (A) Dorsal view of adult mouse brain. (B) Schematic describing the long-range cerebellar circuitry via direct and indirect interactions (dotted lines) throughout the brain and spinal cord. (C) Schematic showing the local cerebellar circuit. The input from the brainstem [via climbing fibers from the inferior olive (IO) and mossy fibers from >35 nuclei] and the spinal cord (via mossy fibers) is collected in the cerebellar cortex by the Purkinje cells (PC) and communicated to the cerebellar output neurons in the cerebellar nuclei (CN) that reside in the white matter. A, anterior; GC, granule cell; GoC, Golgi cell; L, lateral; M, medial; P, posterior; UBC, unipolar brush cell.
In this Review, we provide a comprehensive summary of cerebellum development and repair processes, considering the importance of interactions between different cell lineages in directing the formation of functional circuits, morphogenesis and replenishment of cells following injury. We first introduce the different cell types and lineages. We then describe when and from where they are generated in mice and summarize the array of genetic tools and genomics datasets (Box 1) available for studying cerebellar development. We also explain how cells from different lineages interact with each other to ensure scaling of cell numbers to enable robust circuit function. We then propose how the coordinated interplay between cell types and the distinct cellular properties of progenitors (proliferation, dispersion) as well as mechanical forces dictate morphogenesis during development. We review recent work highlighting how cellular plasticity is stimulated by injury in neonatal mice and changes the developmental genetic programs within lineage hierarchies, and end with recent studies of human cerebellar development that highlight similarities to rodent and new strategies that might account for the evolution of a greatly expanded cerebellum in human.
Box 1. Tools and resources to study cerebellum development and repair.
The cerebellum research community has developed an extensive arsenal of genetic tools to mark cells for fate-mapping studies and generating conditional mutants in specific lineages (Table 1). Additionally, comprehensive single-cell sequencing analyses of the developing mouse cerebellum (Table 2) have provided new insights into the underlying diversity of each cell type and serve as a powerful resource for future hypothesis-driven research.
Genetic tools
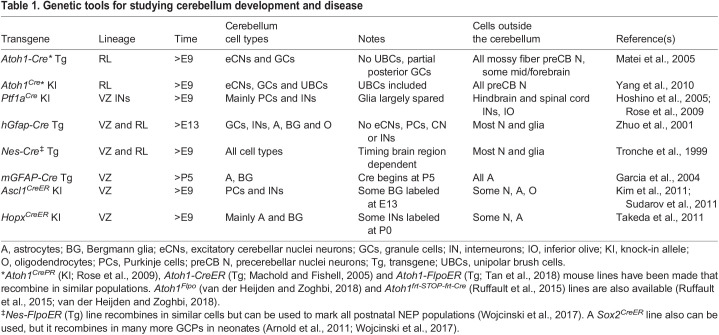
Crucial for understanding the development of any mammalian organ is an array of genetically engineered mouse lines that allow manipulation of specific cell types or lineages and at particular time points. There are well-established mouse lines that express Cre in each of the major cerebellar lineages in the embryo (Atoh1-Cre transgenes for RL, a Ptf1a knock-in line for inhibitory neurons, an mGfap-Cre line for astroglia) as well as lines that hit several or all cell types (an hGFAP-Cre line for neurons and glia generated after E14 and Nes-Cre for all cell types) (Table 1). These lines have been used to generate conditional mutants in new genes that become implicated in cerebellum development. For fate mapping, mouse lines expressing inducible forms of Cre and Flpo have also been generated using promoters from Atoh1 (excitatory lineage), Ascl1 (inhibitory lineage), nestin or Hopx (mainly gliogenic NEPs) (Table 1). For mosaic studies in which a subset of cells within a lineage are mutated and marked with a fluorescent marker, mosaic analysis with double markers (MADM) and mosaic mutant analysis with spatial and temporal control of recombination (MASTR) lines can be used (Lao et al., 2012; Zong et al., 2005).
Genomics resources
Complementary to the genetic tools and elaborate lineage-tracing and fate-mapping experiments, recent advances in genomics, particularly single-cell approaches, continue to unravel the molecular heterogeneity of the cerebellar cell types, particularly the underappreciated diversity within the same lineages or cell types. Furthermore, these approaches have been instrumental in benchmarking transitory states during mouse cerebellar development and upon injury and in identifying cells of origin of cerebellar tumors.
Cerebellar cell types and local circuit organization
The cerebellum is the only brain region that has both an outer layered cortex and an inner nuclear structure. The cerebellar cortex has a folded structure consisting of lobules (Fig. 1B). Within the cortex, excitatory GCs are the most numerous cell type and form a dense layer called the inner granule layer (IGL). Above the IGL is a single layer of large inhibitory neurons, called Purkinje cells (PCs), that extend their elaborate dendrites into an overlying molecular layer (ML) where they are innervated by the T-shaped GC parallel fibers (axons). The ML and IGL also contain several distinct subtypes of locally projecting inhibitory interneurons, and the IGL in the posterior cerebellum has scattered excitatory unipolar brush cells (UBCs) (Fig. 1C). Within the nuclear structure are the cerebellar nuclei (CN), which contain the main output neurons of the cerebellum and local inhibitory interneurons. Thus, the cerebellum has five major neuron types (GCs, excitatory CN neurons, UBCs, PCs, interneurons) with distinct cellular configurations that constitute the local neural circuit (Fig. 1C). In addition to the neurons, the IGL, white matter (made up of myelinated axons) and CN also contain astrocytes. For example, specialized Bergmann glia reside alongside the PCs in the PC layer (also referred to as the Bergmann glia layer; Bayin et al., 2021). Heterogeneity amongst the cerebellar astrocytes is not well understood (Cerrato et al., 2018; Leto et al., 2016; Parmigiani et al., 2015).
In the cerebellar local circuit, the PCs gather incoming information from axons coming into the cerebellum either directly via climbing fibers from the inferior olive or indirectly from mossy fibers that project to the GCs from >35 sites (Fig. 1C). The climbing and mossy fibers relay proprioceptive and sensory information from the muscles and skin of the body, as well as instructions coming from the cerebral cortex. The PCs then project to the CN and the excitatory projection neurons integrate all the incoming information and convey instructions to the rest of the brain (Fig. 1B). Possibly a remnant from more ancient cerebella, the PCs in the posterior-most lobules project outside the cerebellum to the hindbrain vestibular nuclei. In addition, a subset of CN interneurons project to hindbrain nuclei (Judd et al., 2021). Curiously, the excitatory cerebellar nuclei neurons (eCNs) comprise the smallest number of neurons in the cerebellum, despite being the crucial output neurons that connect with the rest of the brain and spinal cord (Sillitoe and Joyner, 2007). This fact is perhaps because they integrate a large array of information into a simpler output signal.
Two distinct cerebellar lineages and their progenitor populations
During mammalian embryonic development, the brain has ventricles surrounded by radially oriented neural progenitor cells called the ventricular zone (VZ), where cells proliferate and give rise to different neurons and glia with spatial and temporal patterning. In general, glia are generated later than neurons, and once a neuron leaves the VZ it stops proliferating and migrates to its final location. The cerebellum is one of the only mouse brain structures that has neural precursors that leave the VZ and continue to proliferate after birth. Furthermore, it has a second progenitor zone situated at the posterior end of the cerebellar anlage called the rhombic lip (RL) that also generates a secondary progenitor zone (Fig. 2A). The VZ and RL maintain distinct lineages and are largely spatially separated from mid-gestation onwards in mice. The VZ gives rise to all the inhibitory neurons, as well as astrocytes and Bergmann glia (astroglia), whereas the RL gives rise exclusively to excitatory neurons (Fig. 2A). The basic helix-loop-helix (bHLH) transcription factors, pancreas-associated transcription factor 1a (PTF1A) and atonal bHLH transcription factor 1 (ATOH1) play antagonistic roles in maintaining the inhibitory and excitatory lineages, respectively (Yamada et al., 2014). We, therefore, discuss the VZ and RL lineages separately.
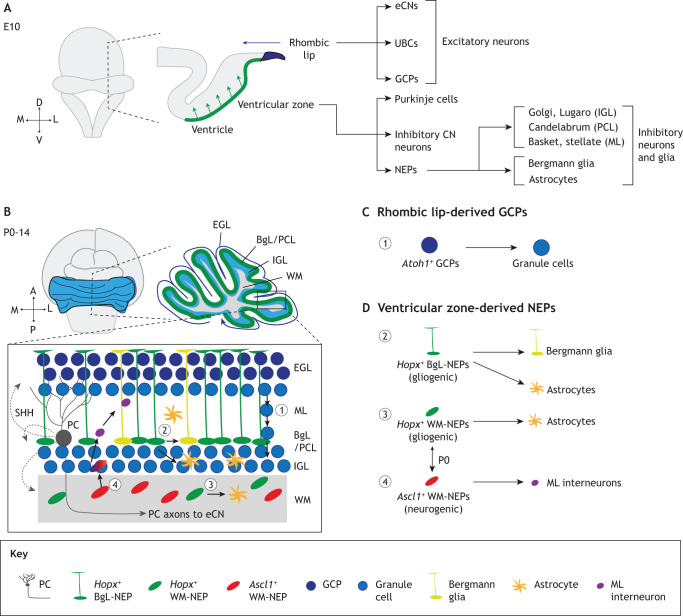
Fig. 2.
Two progenitor zones generate all the cerebellar cell types. (A) During embryonic development, the RL gives rise to excitatory cerebellar nuclei neurons (eCNs), unipolar brush cells (UBCs) and granule cell progenitors (GCPs) and the ventricular zone gives rise to Purkinje cells, inhibitory neurons located in the cerebellar nuclei (CN) and nestin-expressing progenitors (NEPs), which give rise to the inhibitory neurons of the cerebellar cortex and the astroglia. (B-D) During postnatal growth (until ∼P14 in mice), RL-derived GCPs give rise to granule cells (C) and ventricular zone-derived NEPs produce astroglia (Bergmann glia and astrocytes) and molecular layer (ML) interneurons (D). Black arrows show the migration routes of lineages. The double-headed arrows between the Hopx+ and Ascl1+ WM-NEPs denotes the presence of multipotent progenitors at birth, which mostly give rise to Ascl1+ WM-NEPs. Both the GCPs and the three populations of NEPs during postnatal development proliferate in response to SHH secreted (dotted arrows) by Purkinje cells. A, anterior; BgL, Bergmann glia layer; D, dorsal; EGL, external granule layer; IGL, internal granule layer; L, lateral; M, medial; P, posterior; PC, Purkinje cell; PCL, Purkinje cell layer; V, ventral; WM, white matter.
VZ: generation of multiple inhibitory neurons and astrocytes
Embryonic progeny: production of early-born inhibitory interneurons and PCs
The VZ produces PCs and seven interneuron types [interneurons in the CN that include nucleo-olivary projection neurons and deep white matter inhibitory CN neurons; Golgi and Lugaro interneurons in the IGL; basket and stellate cells in the ML (Leto and Rossi, 2012); and candelabrum cells in the PC layer (Osorno et al., 2022)] distinguished by their location and shape (Fig. 2A). Each neuron type is born during a specific period, starting with the PCs and interneurons of the CN that are generated directly from the VZ in the embryo during embryonic day (E) 10 to E13 in mouse (Leto et al., 2016; Leto and Rossi, 2012; Sudarov et al., 2011). Despite being born during a similar period, they have distinct cell shapes, sizes, functions and migratory routes. It is not clear whether a bipotent neural progenitor produces the three inhibitory cell types or whether they derive from distinct locations along the anterior-posterior axis of the VZ. There is evidence that PCs derive mainly from the posterior end of the VZ, from a region marked by expression of oligodendrocyte transcription factor 2 (OLIG2) (Seto et al., 2014) and E-cadherin (cadherin 1) (Mizuhara et al., 2010; Wizeman et al., 2019). As the immature neurons leave the VZ, they express neurogenic transcription factors, including PTF1A and achaete-scute homolog 1 (ASCL1), which guide their development. Strikingly, mice lacking Ptf1a in the brain have very few PCs or interneurons (Hoshino et al., 2005), whereas Ascl1 mutants have a mild reduction of interneurons and an increase in astrocytes (Sudarov et al., 2011). The PCs take up their position under the surface of the cerebellum by E15 and project axons to the CN (Sillitoe et al., 2009). PCs start as a multilayer and, by postnatal day (P) 4, form a single layer sandwiched between the expanding IGL and molecular layer, alongside the Bergmann glia (Fig. 2B) (Hatten and Heintz, 1994; Leto et al., 2016; Sillitoe and Joyner, 2007). Despite the uniform distribution of the PCs throughout the cerebellum, PCs have molecular subtypes that are organized into zones and parasagittal stripes that link to distinct sets of pre- and post-synaptic partners and different firing patterns, respectively, all of which allows the execution of distinct cerebellar functions (reviewed by Cerminara et al., 2015; Dastjerdi et al., 2012; De Zeeuw et al., 2021).
Postnatal progeny: production of molecular layer interneurons and astroglia
At a poorly defined time during mouse embryonic development, nestin-expressing progenitors (NEPs) leave the VZ and settle below the surface of the cerebellum and continue to proliferate after birth (Fleming et al., 2013; Parmigiani et al., 2015). As the layers of the cerebellum start to form at P1, the NEPs become largely segregated into two locations: a layer intermixed with the PCs and a group that resides in the white matter found in the center of each lobule (Fig. 2B,D). Recent single-cell RNA sequencing (scRNA-seq; Table 2) and genetic inducible fate mapping demonstrated that there are three distinct populations of NEPs – one in the PC layer that generates Bergmann glia and astrocytes that settle in the IGL and two in the white matter (Bayin et al., 2021; Cerrato et al., 2018). One NEP subtype in the white matter is neurogenic (Ascl1+) and gives rise only to interneurons of the ML postnatally and possibly the IGL and PC layer at late embryonic stages. The other is mainly gliogenic, Hopx+, and gives rise to astrocytes in the lobule white matter (Bayin et al., 2021; Cerrato et al., 2018). The types of inhibitory neurons in the cerebellar cortex are generated from approximately E17 to P6 and become located in distinct layers (Bayin et al., 2021; Brown et al., 2019; Sudarov et al., 2011). Each inhibitory neuron type is born during a particular time window and settles in an inside-to-outside manner, with the earliest born Golgi interneurons settling in the IGL and each subsequently born taking up a more external position in the ML (Fig. 1C). Interestingly, it appears that the Hopx-expressing NEPs in the white matter are more plastic at earlier stages as they generate a substantial number of interneurons at P0 but mainly astroglia at P5 (Bayin et al., 2021).
Table 2.
Single-cell genomic datasets of mouse cerebellum development
The gliogenic NEPs in the PC layer have a distinct cell shape with a glial process that reaches the cerebellar surface, similar to that of mature Bergmann glia (Fig. 2B,D). These NEPs express both Hopx and growth differentiation factor 10 (Gdf10) and generate astroglia until ∼P10. Whether there are distinct progenitors for the two types of astrocytes is not clear. However, the fact that Bergmann glia and not astrocytes express Gdf10 raises the possibility of a dedicated progenitor for Bergmann glia that expresses Hopx and Gdf10.
Curiously, and unlike other brain regions, cerebellar NEPs do not generate significant numbers of oligodendrocytes (<1% of their progeny during postnatal development) (Bayin et al., 2021). The published work to date indicates that, in mouse, oligodendrocytes are derived from the VZ of the midbrain or hindbrain and that oligodendrocyte progenitors migrate into the white matter of the cerebellum by birth, where they expand and produce mature oligodendrocytes (Grimaldi et al., 2009; Hashimoto et al., 2016; Mecklenburg et al., 2011).
RL: generation of three excitatory cell types
Embryonic progeny: production of excitatory cerebellar nuclei and unipolar brush cells
The cerebellar RL produces the three excitatory cell types (eCNs, GCs and UBCs) and, like the VZ, these different cell types are produced in a time-dependent fashion (Fig. 2A). All excitatory cells are dependent on ATOH1 (Ben-Arie et al., 1997). Furthermore, eCNs and UBCs become post-mitotic when they leave the RL at E9-E12 and E15-E17, respectively. In contrast, the GCs are generated from a dedicated intermediate granule cell progenitor (GCP), which is dependent on ATOH1 and paired box 6 (PAX6) (Swanson et al., 2005; Yeung et al., 2016). GCPs maintain Atoh1 expression and proliferate on the surface of the mouse cerebellum from E14 to P15 (Machold and Fishell, 2005; Sekerková et al., 2004; Wang et al., 2005). The eCNs and UBCs migrate tangentially (anteriorly) under the surface of the cerebellum and eventually express distinct transcription factors, including Meis homeobox 2 (MEIS2) and T-box brain protein 2 (TBR2; EOMES), respectively. The eCNs congregate at the anterior end of the cerebellum for ∼2 days in the ‘nuclear transitory zone’, before the cells gradually assemble into three bi-lateral nuclei inside the cerebellum, either as a result of cell migration and/or accumulation of the other cell types above them.
As with the VZ neural progenitors, it is not clear whether distinct RL progenitors generate the three excitatory neuron populations, or whether there are bi- or tri-potent progenitors. There is growing evidence, however, that the RL has spatially defined subdomains based on gene expression signatures that change during development. The degree to which they represent lineage-restricted progenitors is, nevertheless, unclear (Chizhikov et al., 2010; Khouri-Farah et al., 2022; Yeung and Goldowitz, 2017). It has been recently proposed (based on scRNA-seq and RNA in situ hybridization validation) that a posterior VZ domain, characterized by the expression of reelin (RELN) and HES family bHLH transcription factor 1 (Hes1), feeds cells into the mouse RL. This region was, therefore, called the ‘posterior transitory zone’ (Khouri-Farah et al., 2022). Furthermore, a small population of cells in the Atoh1 lineage (possibly rare MEIS2-expressing eCNs) might be derived from the VZ and migrate radially (Khouri-Farah et al., 2022), which would suggest there is some level of plasticity in VZ cells at mid-gestation.
Postnatal progeny: production of granule neurons
GCPs are unipotent progenitors that migrate anteriorly over the surface of the cerebellum from the RL starting at E13.5 and continue to divide symmetrically to produce either two progenitors or two immature neurons. GCPs and their immediate post-mitotic GCs form the external granule layer (EGL), a transient structure present for ∼2 weeks after birth in mice (Fig. 2B,C). The outer layer of the EGL has the proliferating GCPs, whereas the inner EGL houses the immediate GCs that are extending their axons specifically in the medial-lateral orientation, perpendicular to the PC dendrites with which they synapse. The GC body then migrates a short distance along one of its axon branches before migrating inward along a Bergmann glial fiber to the PC layer and then into the forming IGL. The axon of each GC is therefore T-shaped with the medial-laterally oriented axon called a parallel fiber (Fig. 1C). Because the parallel fibers are laid down on top of each other in the ML, the position of a parallel fiber defines when a GC was born, with the earliest born being immediately above the PC layer (Espinosa and Luo, 2008; Legue et al., 2015; Zong et al., 2005). Importantly, early- and late-born GCs have distinct physiological properties (Shuster et al., 2021). Clonal analyses of embryonic GCPs (E15.5-P1) have not only demonstrated the spatial relationship of parallel fibers and sequence of GC generation, but also that the cell bodies of clones are seemingly randomly located within the inner to outer length of the IGL (Espinosa and Luo, 2008; Legue et al., 2015). Most extraordinarily, however, all the cells in an entire clone of GCPs stop dividing over a 2-day period and then the GCs migrate together to the IGL. In slice cultures, however, GCPs can undergo asymmetric cell divisions similar to VZ cells that produce a neuron and a progenitor (self-renewal) (Merk et al., 2020).
Interdependence of cell lineages: scaling and growth regulation
Scaling of the ratios of different cerebellar neurons with respect to each other is required for proper circuit function and generates the morphology of the cerebellum. The lobules of the cerebellum form in a specific sequence and most growth occurs after birth in mice (Fig. 3A). Signaling between neurons and progenitors is a key driver of cell number scaling and size expansion over the remarkably protracted growth of the cerebellum compared with the rest of the brain. The signaling factors include survival factors (acting on neurons) and proliferative factors (acting on progenitors). It is tempting to speculate, given the interconnected organization of distinct brain regions, that similar rules apply across brain regions, such that one brain region influences the growth/survival of another.
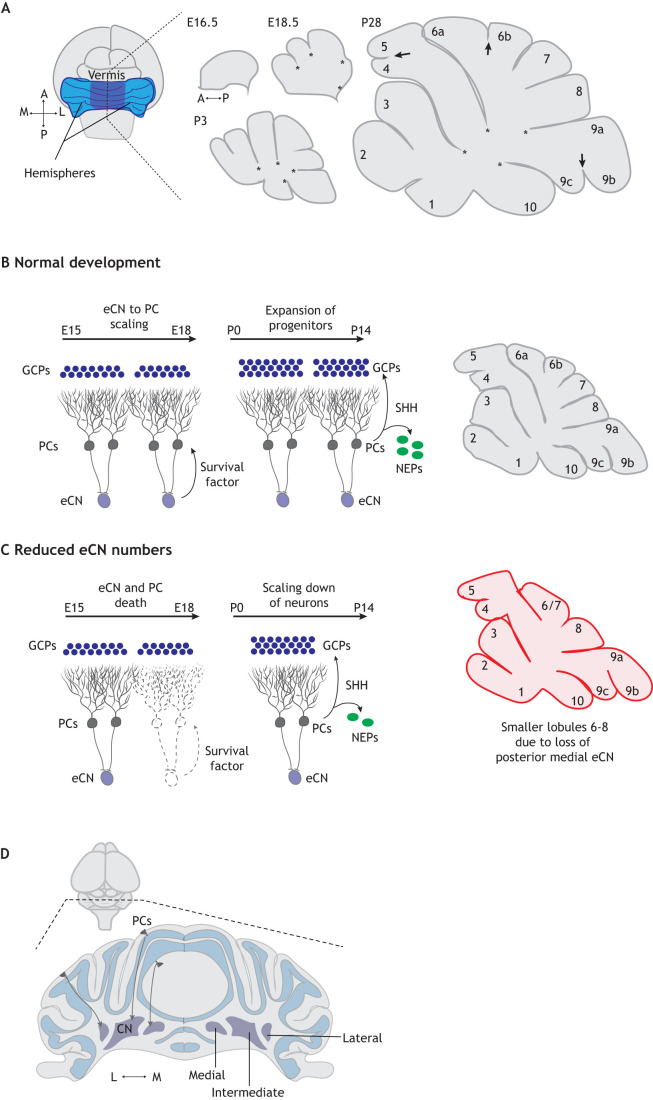
Fig. 3.
Cerebellar morphogenesis and cell scaling. (A) Schematics of midsagittal mouse cerebellar sections illustrating the progression of mouse foliation (asterisks show the anchoring centers and arrows show the secondary lobules). (B) The scaling between the number of excitatory cerebellar nuclei neurons (eCNs) and Purkinje cells (PCs) is thought to be mediated by a survival factor. PCs then scale the number of granule neurons, late-born interneurons and astroglia via SHH, which acts on two precursor populations: granule cell progenitors (GCPs) and nestin-expressing progenitor (NEPs). (C) Reduction in eCN numbers leads to death of their presynaptic PCs within the circuitry unit, leading to location-specific reduction in lobule growth and reduced but scaled granule cell, interneuron and astroglia production in the region. In the example shown, some eCNs in the posterior region of the medial cerebellar nuclei (CN) are missing, which leads to a specific reduction in size of lobules 6-8 in the vermis (compare C with B). (D) Mediolateral distribution of eCNs into three nuclei and location-specific targeting of PCs to CN. A, anterior; L, lateral; M, medial; P, posterior.
Signaling pathways regulating the VZ and RL
The pathways that stimulate proliferation and fate determination of the embryonic VZ and RL, and thereby determine the postnatal growth potential of the cerebellum, are poorly understood. There is evidence, however, that sonic hedgehog (SHH) secreted by the choroid plexus, a structure underneath the cerebellum that produces cerebrospinal fluid, acts on the cerebellar VZ to promote proliferation (Huang et al., 2010). SDF1a (CXCL12) secreted by the adjacent head mesenchyme also seems to promote proliferation of VZ progenitors (Haldipur et al., 2014). There is also evidence that Notch signaling regulates RL and/or VZ specification, as well as proliferation of GCPs (Khouri-Farah et al., 2022; Machold et al., 2007; Solecki et al., 2001). A recent paper has shown that activation of Notch signaling (conditional Notch intracellular domain expression) in the VZ and RL at E8.5 leads to expansion of the posterior Hes1 domain (part of the RL and posterior VZ) and a truncation of the posterior cerebellum (Khouri-Farah et al., 2022). Part of the phenotype involves the RL changing fate to generate Bergmann glia and choroid plexus, presumably at the expense of eCNs and GCPs.
Survival factors expressed by eCNs support PC survival
Once the PCs are born and migrate to the cerebellar cortex, they become dependent on eCNs for their survival, possibly via the action of neurotrophic factors (Fig. 3B). When a subset of eCNs is genetically ablated in mice, a subset of PCs presumed to be their presynaptic partners are lost by E18 (Fig. 3C) (Ahmadzadeh et al., 2020; Willett et al., 2019). The same phenomenon is observed in conditional mutants lacking the engrailed genes (En1/2) in eCNs, where a subset of eCNs die after E15, leading to the loss of their presynaptic PCs. The PC loss does not seem to be due to a lack of neural transmission, as inhibition of PC neural transmission does not cause loss of PCs, despite changes in PC molecular patterning (White and Sillitoe, 2013). Thus, as the neurotrophic hypothesis proposes (Cowan et al., 1984), there appears to be retrograde support for PC viability from eCNs. Consistent with these mouse experiments, cell culture experiments have shown that PC survival can be augmented by neurotrophin application (Lärkfors et al., 1996; Mount et al., 1995). A candidate neurotrophin is brain-derived neurotrophic factor (BDNF) based on its expression (Willett et al., 2019), and the observation that Bdnf and p75NTR (Ngfr; encoding a receptor) null mutants have defects in cerebellum growth and foliation (Carter et al., 2003, 2002; Schwartz et al., 1997). In summary, although PCs and eCNs are generated from separate lineages, PCs are dependent on eCNs for their survival, showing the interdependence of different lineages during development. A reciprocal dependence during development has not been reported.
SHH secreted by PCs supports proliferation of GCPs and NEPs
SHH is expressed by PCs from E18.5 through adulthood. During mouse neonatal development, SHH is a crucial factor stimulating proliferation of both GCPs and NEPs (Fig. 2B) (Corrales et al., 2006, 2004; De Luca et al., 2015; Fleming et al., 2013; Lewis et al., 2004; Parmigiani et al., 2015; Wojcinski et al., 2017). Given that the GCs and interneurons project axons to PCs, it appears that PCs dictate the number of their upstream neurons by regulating the proliferation of their progenitors (GCPs and NEPs) via the SHH they secrete. Indeed, reducing the number of PCs leads to a proportionally scaled decrease in GCs and ML interneurons such that the PC:GC and ML interneuron:PC ratios remain near normal (Willett et al., 2019). One interpretation of these findings is that the cerebellar cortex has tiles comprising a single PC and postnatally derived neurons (and possibly astroglia) in its local circuit that relates to the presence of its target eCN. Whether each PC stimulates the production of GCs and interneurons in its tile is not clear. Furthermore, it is not entirely clear why and how GCPs or NEPs stop responding to SHH and differentiate and whether they have an unlimited capacity to divide. Nevertheless, several factors likely contribute to how GCPs transition from a proliferative state to becoming a neuron, including loss of the cilia required for SHH signaling due to changes in BMP signaling and degradation of ATOH1 protein, as well as the impact of extracellular matrix proteins on cilia formation and SHH signaling (Blaess et al., 2004; Haldipur et al., 2014; Ichikawa-Tomikawa et al., 2012; Ong et al., 2020; Pons et al., 2001).
Regional differences in timing and extent of growth
The different lobules of the cerebellum, along both the anterior-posterior and medial-lateral axes, do not grow at the same rate after birth. In the mouse medial cerebellum (vermis), the central lobules 6 and 7 have a delayed maximum growth phase compared with the other lobules (Fig. 3A) (Legue et al., 2016). This developmental delay is accompanied by a relative delay in the maturation of PCs, and the EGL and ML are thinner in these regions until after P6. One molecular reason for the delay in the growth of lobules 6 and 7 is likely because Shh expression is lower in these lobules compared with other lobules at early stages (Corrales et al., 2004). Lobules 6 and 7 conversely have a thicker EGL at P10-P14 than other vermis lobules; thus, they have a more protracted growth trajectory. Because PCs express SHH, which drives proliferation of GCPs and NEPs, the number of PCs located between each initial fissure should define the growth potential of the intervening lobule and contribute to the patterning of foliation.
Cerebellar morphogenesis involves a combination of physical constraints and differential growth regulation of distinct lineages
The most striking morphological feature of the cerebellum is the series of folds/lobules running along the medial-lateral axis, with distinct folding patterns in the vermis and lateral hemispheres. The basic folding pattern of the cerebellum is conserved across mammals with ten lobules in the vermis (Inouye and Oda, 1980) (Fig. 3A). Each lobule is surrounded by two fissures, and the lobules grow outwards from the initial demarcation of the base of the fissures, referred to as anchoring centers (Fig. 3A) (Legue et al., 2015; Sudarov and Joyner, 2007). Some lobules are subsequently subdivided by shallower ones (arrows at P28 in Fig. 3A) creating sublobules (e.g. 9b and 9c). The difference in the folding patterns between the vermis and hemispheres is, in part, because some lobules are only present in the vermis and do not extend the entire medial-lateral axis. For example, in mice, the anterior folds 1-5 are not present in the hemispheres. In addition, some lobules are larger or have distinct shapes in the hemispheres compared with the vermis. The accumulating data (Engstrom et al., 2018; Lawton et al., 2019; Leffler et al., 2016; Lejeune et al., 2016) indicate that physical forces generated from differential growth in the embryonic cerebellum define the initial position of fissures, and then the signaling between cell lineages described above influences the final shape of each lobule.
Mechanical forces that regulate folding
The anchoring centers form in a specific time series and maintain their relative positions throughout development (Fig. 3A) (Legue et al., 2015; Szulc et al., 2015). The first indication of an anchoring center is an inward thickening of the EGL before the outer surface indents. The surrounding Bergmann glia then orient their glial fibers toward the anchoring center (Sudarov and Joyner, 2007). Similar to differential growth models to explain the folding of the cerebral cortex (Bayly et al., 2013, 2014; Mota and Herculano-Houzel, 2015; Ronan et al., 2014; Tallinen et al., 2014; Van Essen, 2020), several papers have described models of the growth and folding of the cerebellum based on the idea that the EGL expands at a faster rate than the underlying cerebellar cortex (Engstrom et al., 2018; Lawton et al., 2019; Leffler et al., 2016; Lejeune et al., 2016), which after E14 contains all the PCs and an increasing number of NEPs, interneurons and eventually glia. This differential growth leads to a buckling of the EGL, and the buckling points define the base of the fissures that subsequently grow outwards. Each model defines different mechanical properties, and only the model that considers the EGL as fluid rather than elastic and takes into consideration radial and circumferential constraints from axons and/or radial fibers accounts for the initial foliation pattern at E17.5 in mouse (Lawton et al., 2019). Furthermore, there is no obvious cellular prepattern at the initiation of folding (E16.5-E17.5) involving differential proliferation of GCPs or differences in cell size or shape along the anterior-posterior axis. However, by E18.5, SHH is differentially expressed along the anterior-posterior axis, correlating with a relative decrease in proliferation in the central region. Furthermore, Bergmann glia are essential for foliation as they exert constraints on the EGL and provide a ‘highway’ for migration of GCs to the IGL (reviewed by Leung and Li, 2018). Thus, foliation is a complex process involving many mechanical and physical properties, as well as cell-intrinsic mechanisms and signaling.
Impact of GCP clonal growth on morphology of cerebellar lobules
The cerebellum has anisotropic growth oriented in the anterior-posterior axis, illustrated in mice by the P14 cerebellum surface being approximately eight times longer in this axis than medial-laterally. Clonal analyses of embryonic GCPs have revealed that clones in the adult IGL are elongated in the anterior-posterior axis, in part stemming from oriented cell divisions (Espinosa and Luo, 2008; Legue et al., 2015). Furthermore, clones in short versus long lobules have distinct properties, with clones in longer lobules having more cells and a greater anterior-posterior to medial-lateral length ratio (Legue et al., 2015). Interestingly, although the GCPs have a fluid-like behavior with extensive cell mixing and dispersion (Lawton et al., 2019), GCPs do not cross the base of fissures or anchoring centers in the EGL (Legue et al., 2015). Thus, the GCPs within each lobule can be considered as independent developmental units where behaviors such as proliferation rate, number of cell divisions and degree of oriented growth are differentially regulated and influence lobule shape and size.
Impact of general growth regulation mechanisms on the morphology of cerebellar folds
If we consider the folding patterns of different species, then based on models of differential growth, the initial shape of the cerebellar anlage determines where the initial fissures form. In theory, if the number of PCs partitioned between a pair of fissures is different between species, then the size of the intervening lobule should scale to the number of PCs, assuming similar SHH expression levels. Furthermore, the number of eCNs determines the number of PCs that survive after birth, thus eCNs impact the final size and shape of the cerebellum (Fig. 3B,C). Because, in general, there is a correlation between the medial-lateral and anterior-posterior position of PCs and the eCNs they project to in mammals, the distribution of eCNs in each nucleus should influence the shape of lobules (Fig. 3B,C). Thus, many factors determine the precise pattern and shape of lobules in different mammalian species, including physical forces, degree of signaling pathways (e.g. SHH and neurotrophins), the number of early-born neurons in each species (eCNs and PCs) and their spatial distribution and wiring (choice of synaptic partners).
Repair processes in the neonatal cerebellum: parallels with and differences from normal lineage allocation
The human cerebellum undergoes maximal growth during the third trimester and is, therefore, highly sensitive to environmental stress resulting in tissue injury around birth, particularly in premature babies. Thus, it is important to identify the endogenous cellular and molecular processes stimulated in response to injury and to determine the degree to which cerebellar repair can occur. In this respect, recent work has revealed that the neonatal mouse cerebellum has remarkable regenerative potential and lineage plasticity, allowing for replacement of several cell types when they are killed around birth (Altman et al., 1969; Andreotti et al., 2018; Bayin et al., 2018; Wojcinski et al., 2017). However, the endogenous capacity for repair rapidly diminishes after birth, raising the question of whether a basic understanding of neonatal repair will enable development of approaches to stimulate repair after this crucial period. An interesting finding that parallels repair processes in other organs is that the cerebellum does not fully recapitulate the normal developmental series of events but utilizes seemingly alternative processes. Why then have the alternative processes been engraved in our DNA during evolution? One idea is that such mechanisms are utilized as a buffer against the environmental variations that occur during normal development.
Stage-dependent regeneration of mouse neonatal PCs
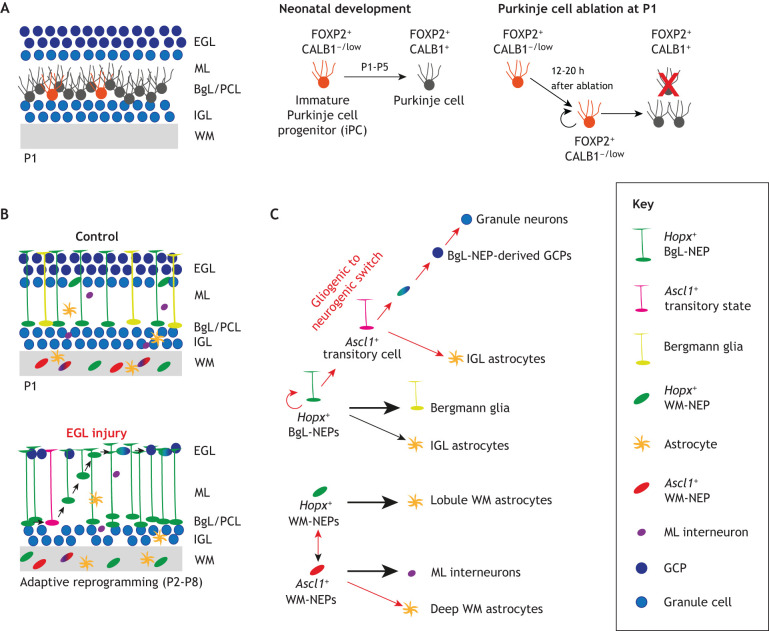
The dogma has been that once a neuron leaves the VZ and stops dividing, it cannot re-initiate proliferation. However, in the mouse neonatal cerebellum, a subpopulation of PCs is immature at P1 and they can proliferate and replace their more mature neighbors if they are killed (Bayin et al., 2018) (Fig. 4A). Only ∼10% of PCs at birth have an immature molecular phenotype (immature Purkinje cell progenitors, iPCs), wherein they express forkhead box protein P2 (FOXP2), which is normally expressed by PCs between E14 and P7, but do not express calbindin 1, a more mature PC marker. Remarkably, the iPCs proliferate within 24 h of death of the more mature PCs. Furthermore, when a genetic approach involving diphtheria toxin is used to kill ∼50% of PCs at P1, the full size of the adult cerebellum is restored. Based on basic motor behavior assays, the newly generated PCs support full functioning of the cerebellum. However, by P5, the iPCs are decreased in number and diminished in their proliferative capacity. Therefore, at P5, iPCs cannot fully replace neighbors that die. Furthermore, there is no evidence that the iPCs proliferate in the absence of injury as they appear instead to fully differentiate. However, it seems plausible that if the number of eCNs that are born in an individual proportionally outnumbers the PCs, then iPCs could sense the imbalance and proliferate. The new PCs would then increase the total amount of SHH and could proportionally increase the number of interneurons, GCs and astroglia in the cerebellar cortex. Finally, these findings indicate that PC molecular and phenotypic heterogeneity includes iPCs during development. The molecular underpinnings of the iPC behaviors stimulated by neonatal injury, however, remain to be discovered.
Fig. 4.
Regenerative mechanisms of the neonatal cerebellum upon injury at birth. (A) During normal development, immature Purkinje cell progenitors (iPCs) slowly mature and become PCs (P1-P5). When ∼50% of Purkinje cells (PCs) are ablated at birth, a dormant iPC can proliferate and replace the lost neurons. iPCs express the early PC marker FOXP2 but have reduced or no expression of the mature PC maker CALB1. (B,C) Upon depletion of GCPs in the external granule layer (EGL) at birth, a normally gliogenic nestin-expressing progenitor (NEP) population that resides in the Bergman glial layer (BgL) undergoes adaptive reprogramming that involves a transitory Ascl1+ cellular state and migration to the EGL to replenish granule cell progenitors (GCPs) that have died. Upon injury, neurogenic-NEPs in the white matter (WM) also show lineage plasticity and generate ectopic astrocytes in the deep white matter (which surrounds the cerebellar nuclei). Red arrows denote activity in response to injury. IGL, internal granule layer; ML, molecular layer; PCL, Purkinje cell layer.
Adaptive reprogramming of Bergmann glial layer NEPs to replenish GCPs after injury
Perhaps less surprisingly than PCs being replenished soon after birth, GCPs can be replenished when they are killed at P1 using irradiation or genetic killing methods involving diphtheria toxin or inhibition of SHH signaling (Altman et al., 1969; Andreotti et al., 2018; Wojcinski et al., 2017, 2019). However, unlike the case of PC replenishment, the final size of the cerebellum does not fully recover after GCP loss. Surprisingly, in all the models of GCP injury, an important part of their replenishment involves the adaptive reprogramming of the Hopx-expressing gliogenic NEPs in the PC layer (Fig. 4B). The cells initially increase their proliferation rate, then they migrate to the EGL (site of injury) and turn off NEP genes (e.g. Sox2 and Nes) and turn on ATOH1 to generate GCPs. Similar to NEPs and GCPs during development, SHH signaling is required for repair of the EGL. Recent scRNA-seq and genetic inducible fate-mapping approaches (Tables 1 and 2) have identified a transitory cellular state that occurs during the NEP-to-GCP fate switch, and showed that the neurogenic gene Ascl1 is crucial for reprogramming (Bayin et al., 2021) (Fig. 4C). It is not clear whether during normal development rare gliogenic NEPs in the PC layer undergo the unusual behavior seen after injury or whether the adaptive reprogramming is specific to injury. Fate mapping using a Nes-FlpoER transgene or HopxCreER has indicated that in the normal cerebellum a very small portion of GCs arise from Nes-expressing cells (Bayin et al., 2021; Wojcinski et al., 2017), but it is not clear if they come from the PC layer. Live imaging of P5 cerebellar slices has detected NEPs migrating from the PC layer to the EGL after in vivo injury, but not in control cerebella, at least during the ∼6-h imaging period. Alternatively, if rare ventricular-zone cells express Atoh1 and migrate to the EGL (Khouri-Farah et al., 2022), perhaps NEPs that are undergoing adaptive reprograming upon injury are recapitulating this program.
Table 1.
Genetic tools for studying cerebellum development and disease
Interestingly, the two NEP subpopulations in the white matter that normally generate interneurons and astrocytes transiently decrease their output of cells ostensibly while they wait for the EGL to be replenished (Bayin et al., 2021; Wojcinski et al., 2017). This behavior is likely linked to the normal processes that regulate proper scaling during development being utilized for regeneration. Finally, the neurogenic NEPs in the white matter also show lineage plasticity upon injury and generate ectopic astrocytes in the deep white matter, where the eCN resides (Fig. 4C). The reason behind this fate switch remains to be explored. However, these results indicate that cells distant to a site of injury can sense the loss of GCPs, or possibly their output neurons, GCs.
The finding that two mouse neonatal cerebellar cell types can be replenished after injury raises many questions. One is whether all cell types can be regenerated? If so, do they arise from progenitors of their own lineage (such as PCs) or is plasticity triggered in a distinct lineage requiring elaborate molecular mechanisms and cell fate switches (such as GCPs)? Equally important, what are the signals that stimulate repair? Is it signals released by the dying cells or more general damage responses, such as reactive oxygen species? Given that death of PCs and GCPs elicit different responses in the surrounding cells that are spared, it appears that at some level the signals released are context specific. Finally, an important question is why the neonatal cerebellum is permissive to regeneration and what the inhibitory mechanisms are that prevent the adult brain from repairing lost cells efficiently after injury. Such inhibitory mechanisms are likely in place to prevent aberrant reprogramming that might cause diseases, including cancer.
Distinct aspects of human cerebellum development and relation to diseases
Compared with mice, much less is known about cerebellum development in primates or humans. A recent review (Haldipur et al., 2022) provides a comprehensive summary of development and diseases of the human cerebellum based on histological and transcriptional analyses; therefore, we focus on specialties that have evolved in human to accommodate the ∼750-fold increase in surface area of the human cerebellum compared with rodent, particularly the hemispheres. The human cerebellum has a more complicated foliation pattern, and the lateral cerebellar nuclei have greatly expanded and contain several distinct subnuclei. The same basic neurons are present, CN neurons and PCs are the earliest born and neurogenesis begins before it does in the cerebral cortex, but the proportions of neurons are distinct in human (Lange, 1975). As in mouse, the human anterior lobes expand earlier than the posterior and this correlates with PC maturation; however, peak proliferation of GCPs occurs in utero (26-32 weeks) rather than postnatally as in rodents, but the human EGL remains in some regions for a year after birth (Haldipur et al., 2021). Curiously, based on scRNA-seq data, the eCNs of the lateral cerebellar nuclei have only one transcriptionally defined neuron subtype compared with two in mice and chicken (Kebschull et al., 2020). Likely to accommodate the greatly expanded cerebellum, two of the progenitor zones are more complex in human (Aldinger et al., 2021; Haldipur et al., 2019, 2021). The VZ has a subventricular zone (SVZ) by 6 post-conceptional weeks, reminiscent of the cerebral cortex of mouse and human. Most astonishing is the morphological changes the RL undergoes starting at 11 post-conceptional weeks, including subdivision by a vascular bed into a so-called RLVZ with more immature progenitors, and an RLSVZ with UBC and GCP precursors. Whether there are parallels between the RLSVZ and the proposed posterior transitory zone in mouse (Khouri-Farah et al., 2022) is not clear. Furthermore, the human RL becomes embedded in the most posterior lobe. The new sub-lineages of the human progenitor zones provide candidates for cells of origin of tumors, especially for the group 3 and 4 subgroups of the cerebellar tumor medulloblastoma, which, compared with the SHH subgroup derived from GCPs, have so far been elusive (Hovestadt et al., 2019; Vladoiu et al., 2019). Finally, the complex RL in human is thought to be crucial for growth of the posterior cerebellum and injury or defects of the structure are responsible for human diseases involving hypoplasia of the posterior vermis. However, the contribution of eCNs to cerebellar growth must also be considered, because they are derived from the RL. An additional question remaining is whether the third progenitor population identified in mouse, the NEPs, also is present in the human cerebellum and, if so, has it evolved to be more complex, do they have regenerative potential for replacing an injured EGL and are there distinct progenitors for interneurons and astroglia as in mouse?
Conclusions and perspectives
The cerebellum has provided insights into how cell-to-cell communication between and within lineages is crucial to ensure the numbers of different cell types are scaled during development and after injury, and generate the complex foliation pattern that underlies circuit integrity. The degree to which new neurons generated after injury to the mouse neonatal cerebellum integrate normally into the existing neural circuits and take on the robust molecular heterogeneity remains to be determined. In order to study the relevance of the cellular complexity of the RL and VZ in human, it is crucial to further develop and utilize model systems, in particular human (and mouse) pluripotent stem cell-derived 3D cerebellar organoids (Ballabio et al., 2020; Behesti et al., 2021; Buchholz et al., 2020; Muguruma et al., 2015; Nayler et al., 2021; Seto et al., 2014; Silva et al., 2020a,b). To date, the systems have not documented the inter-lineage and cell-to-cell interactions required in vivo for expansion of different progenitors and survival of PCs, thus much remains to be studied. Finally, if survival of eCNs is dependent on the presence of their target neurons in the midbrain/forebrain, it will be interesting to determine whether some human cerebellar hypoplasias are secondary to injury during in utero development to extra-cerebellar sites (Aldinger et al., 2019; Limperopoulos et al., 2014, 2005).
Acknowledgements
A.L.J. would like to thank past and present members of the laboratory for all the enlightening discussions and insights provided into cerebellum development that are the foundation for writing this review. We thank Anjana Krishnamurthy, Salsa El Nagar and Andrew Lee for helpful comments on the manuscript.
Footnotes
Funding
A.L.J. has been supported recently by grants from the National Institute of Neurological Disorders and Stroke (R01NS092096), the National Institute of Mental Health (R37MH085726) and the National Cancer Institute (R01CA192176), the Functional Genomics Initiative of Memorial Sloan-Kettering Cancer Center (GC24221), the Tri-Institutional Stem Cell Initiative (Memorial Sloan-Kettering Cancer Center, Rockefeller University and Weill Cornell Medical College; GC259064) and a National Cancer Institute Cancer Center Support Grant (P30 CA008748-48). N.S.B. was supported by postdoctoral fellowships from New York State Stem Cell Science (NYSTEM; C32599GG) and the National Institute of Neurological Disorders and Stroke (K99/R00 NS112605-01). Deposited in PMC for release after 12 months.
References
- Ahmadzadeh, E., Bayin, N. S., Qu, X., Singh, A., Madisen, L., Stephen, D., Zeng, H., Joyner, A. L. and Rosello-Diez, A. (2020). A collection of genetic mouse lines and related tools for inducible and reversible intersectional mis-expression. Development 147, dev186650. 10.1242/dev.186650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldinger, K. A., Timms, A. E., Thomson, Z., Mirzaa, G. M., Bennett, J. T., Rosenberg, A. B., Roco, C. M., Hirano, M., Abidi, F., Haldipur, P.et al. (2019). Redefining the etiologic landscape of cerebellar malformations. Am. J. Hum. Genet. 105, 606-615. 10.1016/j.ajhg.2019.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldinger, K. A., Thomson, Z., Phelps, I. G., Haldipur, P., Deng, M., Timms, A. E., Hirano, M., Santpere, G., Roco, C., Rosenberg, A. B.et al. (2021). Spatial and cell type transcriptional landscape of human cerebellar development. Nat. Neurosci. 24, 1163-1175. 10.1038/s41593-021-00872-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman, J., Anderson, W. J. and Wright, K. A. (1969). Early effects of X-irradiation of the cerebellum in infant rats: decimation and reconstitution of the external granular layer. Exp. Neurol. 24, 196-216. 10.1016/0014-4886(69)90015-6 [DOI] [PubMed] [Google Scholar]
- Andreotti, J. P., Prazeres, P. H. D. M., Magno, L. A. V., Romano-Silva, M. A., Mintz, A. and Birbrair, A. (2018). Neurogenesis in the postnatal cerebellum after injury. Int. J. Dev. Neurosci. 67, 33-36. 10.1016/j.ijdevneu.2018.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold, K., Sarkar, A., Yram, M. A., Polo, J. M., Bronson, R., Sengupta, S., Seandel, M., Geijsen, N. and Hochedlinger, K. (2011). Sox2+ adult stem and progenitor cells are important for tissue regeneration and survival of mice. Cell Stem Cell 9, 317-329. 10.1016/j.stem.2011.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo, F. A., Carvalho, L. R., Grinberg, L. T., Farfel, J. M., Ferretti, R. E., Leite, R. E., Jacob Filho, W., Lent, R. and Herculano-Houzel, S. (2009). Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J. Comp. Neurol. 513, 532-541. 10.1002/cne.21974 [DOI] [PubMed] [Google Scholar]
- Ballabio, C., Anderle, M., Gianesello, M., Lago, C., Miele, E., Cardano, M., Aiello, G., Piazza, S., Caron, D., Gianno, F.et al. (2020). Modeling medulloblastoma in vivo and with human cerebellar organoids. Nat. Commun. 11, 583. 10.1038/s41467-019-13989-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayin, N. S., Wojcinski, A., Mourton, A., Saito, H., Suzuki, N. and Joyner, A. L. (2018). Age-dependent dormant resident progenitors are stimulated by injury to regenerate Purkinje neurons. eLife 7, e39879. 10.7554/eLife.39879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayin, N. S., Mizrak, D., Stephen, D. N., Lao, Z., Sims, P. A. and Joyner, A. L. (2021). Injury-induced ASCL1 expression orchestrates a transitory cell state required for repair of the neonatal cerebellum. Sci. Adv. 7, eabj1598. 10.1126/sciadv.abj1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayly, P. V., Okamoto, R. J., Xu, G., Shi, Y. and Taber, L. A. (2013). A cortical folding model incorporating stress-dependent growth explains gyral wavelengths and stress patterns in the developing brain. Phys. Biol. 10, 016005. 10.1088/1478-3975/10/1/016005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayly, P. V., Taber, L. A. and Kroenke, C. D. (2014). Mechanical forces in cerebral cortical folding: a review of measurements and models. J. Mech. Behav. Biomed. Mater. 29, 568-581. 10.1016/j.jmbbm.2013.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behesti, H., Kocabas, A., Buchholz, D. E., Carroll, T. S. and Hatten, M. E. (2021). Altered temporal sequence of transcriptional regulators in the generation of human cerebellar granule cells. eLife 10, e67074. 10.7554/eLife.67074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Arie, N., Bellen, H. J., Armstrong, D. L., McCall, A. E., Gordadze, P. R., Guo, Q., Matzuk, M. M. and Zoghbi, H. Y. (1997). Math1 is essential for genesis of cerebellar granule neurons. Nature 390, 169-172. 10.1038/36579 [DOI] [PubMed] [Google Scholar]
- Blaess, S., Graus-Porta, D., Belvindrah, R., Radakovits, R., Pons, S., Littlewood-Evans, A., Senften, M., Guo, H., Li, Y., Miner, J. H.et al. (2004). β1-integrins are critical for cerebellar granule cell precursor proliferation. J. Neurosci. 24, 3402-3412. 10.1523/JNEUROSCI.5241-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, A. M., Arancillo, M., Lin, T., Catt, D. R., Zhou, J., Lackey, E. P., Stay, T. L., Zuo, Z., White, J. J. and Sillitoe, R. V. (2019). Molecular layer interneurons shape the spike activity of cerebellar Purkinje cells. Sci. Rep. 9, 1742. 10.1038/s41598-018-38264-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz, D. E., Carroll, T. S., Kocabas, A., Zhu, X., Behesti, H., Faust, P. L., Stalbow, L., Fang, Y. and Hatten, M. E. (2020). Novel genetic features of human and mouse Purkinje cell differentiation defined by comparative transcriptomics. Proc. Natl. Acad. Sci. USA 117, 15085-15095. 10.1073/pnas.2000102117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caligiore, D., Pezzulo, G., Baldassarre, G., Bostan, A. C., Strick, P. L., Doya, K., Helmich, R. C., Dirkx, M., Houk, J., Jorntell, H.et al. (2017). Consensus paper: towards a systems-level view of cerebellar function: the interplay between cerebellum, basal ganglia, and cortex. Cerebellum 16, 203-229. 10.1007/s12311-016-0763-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter, A. R., Chen, C., Schwartz, P. M. and Segal, R. A. (2002). Brain-derived neurotrophic factor modulates cerebellar plasticity and synaptic ultrastructure. J. Neurosci. 22, 1316-1327. 10.1523/JNEUROSCI.22-04-01316.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter, A. R., Berry, E. M. and Segal, R. A. (2003). Regional expression of p75NTR contributes to neurotrophin regulation of cerebellar patterning. Mol. Cell. Neurosci. 22, 1-13. 10.1016/S1044-7431(02)00015-5 [DOI] [PubMed] [Google Scholar]
- Carter, R. A., Bihannic, L., Rosencrance, C., Hadley, J. L., Tong, Y., Phoenix, T. N., Natarajan, S., Easton, J., Northcott, P. A. and Gawad, C. (2018). A single-cell transcriptional atlas of the developing murine cerebellum. Curr. Biol. 28, 2910-2920.e2. 10.1016/j.cub.2018.07.062 [DOI] [PubMed] [Google Scholar]
- Cerminara, N. L., Lang, E. J., Sillitoe, R. V. and Apps, R. (2015). Redefining the cerebellar cortex as an assembly of non-uniform Purkinje cell microcircuits. Nat. Rev. Neurosci. 16, 79-93. 10.1038/nrn3886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerrato, V., Parmigiani, E., Figueres-Onate, M., Betizeau, M., Aprato, J., Nanavaty, I., Berchialla, P., Luzzati, F., de'Sperati, C., Lopez-Mascaraque, L.et al. (2018). Multiple origins and modularity in the spatiotemporal emergence of cerebellar astrocyte heterogeneity. PLoS Biol. 16, e2005513. 10.1371/journal.pbio.2005513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chizhikov, V. V., Lindgren, A. G., Mishima, Y., Roberts, R. W., Aldinger, K. A., Miesegaes, G. R., Currle, D. S., Monuki, E. S. and Millen, K. J. (2010). Lmx1a regulates fates and location of cells originating from the cerebellar rhombic lip and telencephalic cortical hem. Proc. Natl. Acad. Sci. USA 107, 10725-10730. 10.1073/pnas.0910786107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrales, J. D., Rocco, G. L., Blaess, S., Guo, Q. and Joyner, A. L. (2004). Spatial pattern of sonic hedgehog signaling through Gli genes during cerebellum development. Development 131, 5581-5590. 10.1242/dev.01438 [DOI] [PubMed] [Google Scholar]
- Corrales, J. D., Blaess, S., Mahoney, E. M. and Joyner, A. L. (2006). The level of sonic hedgehog signaling regulates the complexity of cerebellar foliation. Development 133, 1811-1821. 10.1242/dev.02351 [DOI] [PubMed] [Google Scholar]
- Cowan, W. M., Fawcett, J. W., O'Leary, D. D. and Stanfield, B. B. (1984). Regressive events in neurogenesis. Science 225, 1258-1265. 10.1126/science.6474175 [DOI] [PubMed] [Google Scholar]
- Dastjerdi, F. V., Consalez, G. G. and Hawkes, R. (2012). Pattern formation during development of the embryonic cerebellum. Front. Neuroanat. 6, 10. 10.3389/fnana.2012.00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca, A., Parmigiani, E., Tosatto, G., Martire, S., Hoshino, M., Buffo, A., Leto, K. and Rossi, F. (2015). Exogenous sonic hedgehog modulates the pool of GABAergic interneurons during cerebellar development. Cerebellum 14, 72-85. 10.1007/s12311-014-0596-x [DOI] [PubMed] [Google Scholar]
- De Zeeuw, C. I., Lisberger, S. G. and Raymond, J. L. (2021). Diversity and dynamism in the cerebellum. Nat. Neurosci. 24, 160-167. 10.1038/s41593-020-00754-9 [DOI] [PubMed] [Google Scholar]
- Engstrom, T. A., Zhang, T., Lawton, A. K., Joyner, A. L. and Schwarz, J. M. (2018). Buckling without bending: a new paradigm in morphogenesis. Phys. Rev. X 8, 041053. 10.1103/PhysRevX.8.041053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa, J. S. and Luo, L. (2008). Timing neurogenesis and differentiation: insights from quantitative clonal analyses of cerebellar granule cells. J. Neurosci. 28, 2301-2312. 10.1523/JNEUROSCI.5157-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming, J. T., He, W., Hao, C., Ketova, T., Pan, F. C., Wright, C. C., Litingtung, Y. and Chiang, C. (2013). The Purkinje neuron acts as a central regulator of spatially and functionally distinct cerebellar precursors. Dev. Cell 27, 278-292. 10.1016/j.devcel.2013.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia, A. D., Doan, N. B., Imura, T., Bush, T. G. and Sofroniew, M. V. (2004). GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat. Neurosci. 7, 1233-1241. 10.1038/nn1340 [DOI] [PubMed] [Google Scholar]
- Grimaldi, P., Parras, C., Guillemot, F., Rossi, F. and Wassef, M. (2009). Origins and control of the differentiation of inhibitory interneurons and glia in the cerebellum. Dev. Biol. 328, 422-433. 10.1016/j.ydbio.2009.02.008 [DOI] [PubMed] [Google Scholar]
- Haldipur, P., Gillies, G. S., Janson, O. K., Chizhikov, V. V., Mithal, D. S., Miller, R. J. and Millen, K. J. (2014). Foxc1 dependent mesenchymal signalling drives embryonic cerebellar growth. eLife 3, e03962. 10.7554/eLife.03962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldipur, P., Aldinger, K. A., Bernardo, S., Deng, M., Timms, A. E., Overman, L. M., Winter, C., Lisgo, S. N., Razavi, F., Silvestri, E.et al. (2019). Spatiotemporal expansion of primary progenitor zones in the developing human cerebellum. Science 366, 454-460. 10.1126/science.aax7526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldipur, P., Bernardo, S., Aldinger, K. A., Sivakumar, T., Millman, J., Sjoboen, A. H., Dang, D., Dubocanin, D., Deng, M., Timms, A. E.et al. (2021). Evidence of disrupted rhombic lip development in the pathogenesis of Dandy-Walker malformation. Acta Neuropathol. 142, 761-776. 10.1007/s00401-021-02355-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldipur, P., Millen, K. J. and Aldinger, K. A. (2022). Human cerebellar development and transcriptomics: implications for neurodevelopmental disorders. Annu. Rev. Neurosci. 45, 515-531. 10.1146/annurev-neuro-111020-091953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto, R., Hori, K., Owa, T., Miyashita, S., Dewa, K., Masuyama, N., Sakai, K., Hayase, Y., Seto, Y., Inoue, Y. U.et al. (2016). Origins of oligodendrocytes in the cerebellum, whose development is controlled by the transcription factor, Sox9. Mech. Dev. 140, 25-40. 10.1016/j.mod.2016.02.004 [DOI] [PubMed] [Google Scholar]
- Hatten, M. E. (2020). Adding cognitive connections to the cerebellum. Science 370, 1411-1412. 10.1126/science.abf4483 [DOI] [PubMed] [Google Scholar]
- Hatten, M. E. and Heintz, N. (1994). Mechanisms of neural patterning and specification in the developing cerebellum. Annu. Rev. Neurosci. 18, 385-408. 10.1146/annurev.ne.18.030195.002125 [DOI] [PubMed] [Google Scholar]
- Hoshino, M., Nakamura, S., Mori, K., Kawauchi, T., Terao, M., Nishimura, Y. V., Fukuda, A., Fuse, T., Matsuo, N., Sone, M.et al. (2005). Ptf1a, a bHLH transcriptional gene, defines GABAergic neuronal fates in cerebellum. Neuron 47, 201-213. 10.1016/j.neuron.2005.06.007 [DOI] [PubMed] [Google Scholar]
- Hovestadt, V., Smith, K. S., Bihannic, L., Filbin, M. G., Shaw, M. L., Baumgartner, A., DeWitt, J. C., Groves, A., Mayr, L., Weisman, H. R.et al. (2019). Resolving medulloblastoma cellular architecture by single-cell genomics. Nature 572, 74-79. 10.1038/s41586-019-1434-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, X., Liu, J., Ketova, T., Fleming, J. T., Grover, V. K., Cooper, M. K., Litingtung, Y. and Chiang, C. (2010). Transventricular delivery of Sonic hedgehog is essential to cerebellar ventricular zone development. Proc. Natl. Acad. Sci. USA 107, 8422-8427. 10.1073/pnas.0911838107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa-Tomikawa, N., Ogawa, J., Douet, V., Xu, Z., Kamikubo, Y., Sakurai, T., Kohsaka, S., Chiba, H., Hattori, N., Yamada, Y.et al. (2012). Laminin α1 is essential for mouse cerebellar development. Matrix Biol. 31, 17-28. 10.1016/j.matbio.2011.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye, M. and Oda, S.-I. (1980). Strain-specific variations in the folial pattern of the mouse cerebellum. J. Comp. Neurol. 190, 357-362. 10.1002/cne.901900209 [DOI] [PubMed] [Google Scholar]
- Judd, E. N., Lewis, S. M. and Person, A. L. (2021). Diverse inhibitory projections from the cerebellar interposed nucleus. eLife 10, e66231. 10.7554/eLife.66231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebschull, J. M., Richman, E. B., Ringach, N., Friedmann, D., Albarran, E., Kolluru, S. S., Jones, R. C., Allen, W. E., Wang, Y., Cho, S. W.et al. (2020). Cerebellar nuclei evolved by repeatedly duplicating a conserved cell-type set. Science 370, eabd5059. 10.1126/science.abd5059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khouri-Farah, N., Guo, Q., Morgan, K., Shin, J. and Li, J. Y. H. (2022). Integrated single-cell transcriptomic and epigenetic study of cell state transition and lineage commitment in embryonic mouse cerebellum. Sci. Adv. 8, eabl9156. 10.1126/sciadv.abl9156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, E. J., Ables, J. L., Dickel, L. K., Eisch, A. J. and Johnson, J. E. (2011). Ascl1 (Mash1) defines cells with long-term neurogenic potential in subgranular and subventricular zones in adult mouse brain. PLoS One 6, e18472. 10.1371/journal.pone.0018472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange, W. (1975). Cell number and cell density in the cerebellar cortex of man and some other mammals. Cell Tissue Res. 157, 115-124. 10.1007/BF00223234 [DOI] [PubMed] [Google Scholar]
- Lao, Z., Raju, G. P., Bai, C. B. and Joyner, A. L. (2012). MASTR: a technique for mosaic mutant analysis with spatial and temporal control of recombination using conditional floxed alleles in mice. Cell Rep. 2, 386-396. 10.1016/j.celrep.2012.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lärkfors, L., Lindsay, R. M. and Alderson, R. F. (1996). Characterization of the responses of Purkinje cells to neurotrophin treatment. J. Neurochem. 66, 1362-1373. 10.1046/j.1471-4159.1996.66041362.x [DOI] [PubMed] [Google Scholar]
- Lawton, A. K., Engstrom, T., Rohrbach, D., Omura, M., Turnbull, D. H., Mamou, J., Zhang, T., Schwarz, J. M. and Joyner, A. L. (2019). Cerebellar folding is initiated by mechanical constraints on a fluid-like layer without a cellular pre-pattern. eLife 8, e45019. 10.7554/eLife.45019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffler, S. R., Legue, E., Aristizabal, O., Joyner, A. L., Peskin, C. S. and Turnbull, D. H. (2016). A mathematical model of granule cell generation during mouse cerebellum development. Bull. Math. Biol. 78, 859-878. 10.1007/s11538-016-0163-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legue, E., Riedel, E. and Joyner, A. L. (2015). Clonal analysis reveals granule cell behaviors and compartmentalization that determine the folded morphology of the cerebellum. Development 142, 1661-1671. 10.1242/dev.120287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legue, E., Gottshall, J. L., Jaumouille, E., Rosello-Diez, A., Shi, W., Barraza, L. H., Washington, S., Grant, R. L. and Joyner, A. L. (2016). Differential timing of granule cell production during cerebellum development underlies generation of the foliation pattern. Neural Dev. 11, 17. 10.1186/s13064-016-0072-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejeune, E., Javili, A., Weickenmeier, J., Kuhl, E. and Linder, C. (2016). Tri-layer wrinkling as a mechanism for anchoring center initiation in the developing cerebellum. Soft Mat. 12, 5613-5620. 10.1039/c6sm00526h [DOI] [PubMed] [Google Scholar]
- Leto, K. and Rossi, F. (2012). Specification and differentiation of cerebellar GABAergic neurons. Cerebellum 11, 434-435. 10.1007/s12311-011-0324-8 [DOI] [PubMed] [Google Scholar]
- Leto, K., Arancillo, M., Becker, E. B., Buffo, A., Chiang, C., Ding, B., Dobyns, W. B., Dusart, I., Haldipur, P., Hatten, M. E.et al. (2016). Consensus paper: cerebellar development. Cerebellum 15, 789-828. 10.1007/s12311-015-0724-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung, A.W. and Li, J.Y.H. (2018). The molecular pathway regulating Bergmann glia and folia generation in the cerebellum. Cerebellum 17, 42-48. 10.1007/s12311-017-0904-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, P. M., Gritli-Linde, A., Smeyne, R., Kottmann, A. and McMahon, A. P. (2004). Sonic hedgehog signaling is required for expansion of granule neuron precursors and patterning of the mouse cerebellum. Dev. Biol. 270, 393-410. 10.1016/j.ydbio.2004.03.007 [DOI] [PubMed] [Google Scholar]
- Limperopoulos, C., Soul, J. S., Haidar, H., Huppi, P. S., Bassan, H., Warfield, S. K., Robertson, R. L., Moore, M., Akins, P., Volpe, J. J.. et al. (2005). Impaired trophic interactions between the cerebellum and the cerebrum among preterm infants. Pediatrics 116, 844-850. 10.1542/peds.2004-2282 [DOI] [PubMed] [Google Scholar]
- Limperopoulos, C., Chilingaryan, G., Sullivan, N., Guizard, N., Robertson, R. L. and du Plessis, A. J. (2014). Injury to the premature cerebellum: outcome is related to remote cortical development. Cereb. Cortex 24, 728-736. 10.1093/cercor/bhs354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machold, R. and Fishell, G. (2005). Math1 is expressed in temporally discrete pools of cerebellar rhombic-lip neural progenitors. Neuron 48, 17-24. 10.1016/j.neuron.2005.08.028 [DOI] [PubMed] [Google Scholar]
- Machold, R. P., Kittell, D. J. and Fishell, G. J. (2007). Antagonism between Notch and bone morphogenetic protein receptor signaling regulates neurogenesis in the cerebellar rhombic lip. Neural Dev. 2, 5. 10.1186/1749-8104-2-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek, S., Siegel, J. S., Gordon, E. M., Raut, R. V., Gratton, C., Newbold, D. J., Ortega, M., Laumann, T. O., Adeyemo, B., Miller, D. B.et al. (2018). Spatial and temporal organization of the individual human cerebellum. Neuron 100, 977-993.e7. 10.1016/j.neuron.2018.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matei, V., Pauley, S., Kaing, S., Rowitch, D., Beisel, K. W., Morris, K., Feng, F., Jones, K., Lee, J. and Fritzsch, B. (2005). Smaller inner ear sensory epithelia in Neurog1 null mice are related to earlier hair cell cycle exit. Dev. Dyn. 234, 633-650. 10.1002/dvdy.20551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecklenburg, N., Garcia-López, R., Puelles, E., Sotelo, C. and Martinez, S. (2011). Cerebellar oligodendroglial cells have a mesencephalic origin. Glia 59, 1946-1957. 10.1002/glia.21236 [DOI] [PubMed] [Google Scholar]
- Merk, D. J., Zhou, P., Cohen, S. M., Pazyra-Murphy, M. F., Hwang, G. H., Rehm, K. J., Alfaro, J., Reid, C. M., Zhao, X., Park, E.et al. (2020). The Eya1 phosphatase mediates Shh-driven symmetric cell division of cerebellar granule cell precursors. Dev. Neurosci. 42, 170-186. 10.1159/000512976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuhara, E., Minaki, Y., Nakatani, T., Kumai, M., Inoue, T., Muguruma, K., Sasai, Y. and Ono, Y. (2010). Purkinje cells originate from cerebellar ventricular zone progenitors positive for Neph3 and E-cadherin. Dev. Biol. 338, 202-214. 10.1016/j.ydbio.2009.11.032 [DOI] [PubMed] [Google Scholar]
- Mota, B. and Herculano-Houzel, S. (2015). Cortical folding scales universally with surface area and thickness, not number of neurons. Science 349, 74-77. 10.1126/science.aaa9101 [DOI] [PubMed] [Google Scholar]
- Mount, H. T., Dean, D. O., Alberch, J., Dreyfus, C. F. and Black, I. B. (1995). Glial cell line-derived neurotrophic factor promotes the survival and morphologic differentiation of Purkinje cells. Proc. Natl. Acad. Sci. USA 92, 9092-9096. 10.1073/pnas.92.20.9092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muguruma, K., Nishiyama, A., Kawakami, H., Hashimoto, K. and Sasai, Y. (2015). Self-organization of polarized cerebellar tissue in 3D culture of human pluripotent stem cells. Cell Rep. 10, 537-550. 10.1016/j.celrep.2014.12.051 [DOI] [PubMed] [Google Scholar]
- Nayler, S., Agarwal, D., Curion, F., Bowden, R. and Becker, E. B. E. (2021). High-resolution transcriptional landscape of xeno-free human induced pluripotent stem cell-derived cerebellar organoids. Sci. Rep. 11, 12959. 10.1038/s41598-021-91846-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong, T., Trivedi, N., Wakefield, R., Frase, S. and Solecki, D. J. (2020). Siah2 integrates mitogenic and extracellular matrix signals linking neuronal progenitor ciliogenesis with germinal zone occupancy. Nat. Commun. 11, 5312. 10.1038/s41467-020-19063-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorno, T., Rudolph, S., Nguyen, T., Kozareva, V., Nadaf, N. M., Norton, A., Macosko, E. Z., Lee, W. A. and Regehr, W. G. (2022). Candelabrum cells are ubiquitous cerebellar cortex interneurons with specialized circuit properties. Nat. Neurosci. 25, 702-713. 10.1038/s41593-022-01057-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmigiani, E., Leto, K., Rolando, C., Figueres-Onate, M., Lopez-Mascaraque, L., Buffo, A. and Rossi, F. (2015). Heterogeneity and bipotency of astroglial-like cerebellar progenitors along the interneuron and glial lineages. J. Neurosci. 35, 7388-7402. 10.1523/jneurosci.5255-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisano, T. J., Dhanerawala, Z. M., Kislin, M., Bakshinskaya, D., Engel, E. A., Hansen, E. J., Hoag, A. T., Lee, J., de Oude, N. L., Venkataraju, K. U.et al. (2021). Homologous organization of cerebellar pathways to sensory, motor, and associative forebrain. Cell Rep. 36, 109721. 10.1016/j.celrep.2021.109721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons, S., Trejo, J. L., Martinez-Morales, J. R. and Marti, E. (2001). Vitronectin regulates Sonic hedgehog activity during cerebellum development through CREB phosphorylation. Development 128, 1481-1492. 10.1242/dev.128.9.1481 [DOI] [PubMed] [Google Scholar]
- Rakic, P. and Sidman, R. L. (1970). Histogenesis of cortical layers in human cerebellum, particularly the lamina dissecans. J. Comp. Neurol. 139, 473-500. 10.1002/cne.901390407 [DOI] [PubMed] [Google Scholar]
- Ronan, L., Voets, N., Rua, C., Alexander-Bloch, A., Hough, M., Mackay, C., Crow, T. J., James, A., Giedd, J. N. and Fletcher, P. C. (2014). Differential tangential expansion as a mechanism for cortical gyrification. Cereb. Cortex 24, 2219-2228. 10.1093/cercor/bht082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose, M. F., Ahmad, K. A., Thaller, C. and Zoghbi, H. Y. (2009). Excitatory neurons of the proprioceptive, interoceptive, and arousal hindbrain networks share a developmental requirement for Math1. Proc. Natl. Acad. Sci. USA 106, 22462-22467. 10.1073/pnas.0911579106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffault, P. L., D'Autreaux, F., Hayes, J. A., Nomaksteinsky, M., Autran, S., Fujiyama, T., Hoshino, M., Hagglund, M., Kiehn, O., Brunet, J. F.et al. (2015). The retrotrapezoid nucleus neurons expressing Atoh1 and Phox2b are essential for the respiratory response to CO2. eLife 4, e07051. 10.7554/eLife.07051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarropoulos, I., Sepp, M., Fromel, R., Leiss, K., Trost, N., Leushkin, E., Okonechnikov, K., Joshi, P., Giere, P., Kutscher, L. M.et al. (2021). Developmental and evolutionary dynamics of cis-regulatory elements in mouse cerebellar cells. Science 373, eabg4696. 10.1126/science.abg4696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann, J. D. (2019). The cerebellum and cognition. Neurosci. Lett. 688, 62-75. 10.1016/j.neulet.2018.07.005 [DOI] [PubMed] [Google Scholar]
- Schwartz, P. M., Borghesani, P. R., Levy, R. L., Pomeroy, S. L. and Segal, R. A. (1997). Abnormal cerebellar development and foliation in BDNF-/- mice reveals a role for neurotrophins in CNS patterning. Neuron 19, 269-281. 10.1016/S0896-6273(00)80938-1 [DOI] [PubMed] [Google Scholar]
- Sekerková, G., Ilijic, E. and Mugnaini, E. (2004). Time of origin of unipolar brush cells in the rat cerebellum as observed by prenatal bromodeoxyuridine labeling. Neuroscience 127, 845-858. 10.1016/j.neuroscience.2004.05.050 [DOI] [PubMed] [Google Scholar]
- Seto, Y., Nakatani, T., Masuyama, N., Taya, S., Kumai, M., Minaki, Y., Hamaguchi, A., Inoue, Y. U., Inoue, T., Miyashita, S.et al. (2014). Temporal identity transition from Purkinje cell progenitors to GABAergic interneuron progenitors in the cerebellum. Nat. Commun. 5, 3337. 10.1038/ncomms4337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuster, S. A., Wagner, M. J., Pan-Doh, N., Ren, J., Grutzner, S. M., Beier, K. T., Kim, T. H., Schnitzer, M. J. and Luo, L. (2021). The relationship between birth timing, circuit wiring, and physiological response properties of cerebellar granule cells. Proc. Natl. Acad. Sci. USA 118, e2101826118. 10.1073/pnas.2101826118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillitoe, R. V. and Joyner, A. L. (2007). Morphology, molecular codes, and circuitry produce the three-dimensional complexity of the cerebellum. Annu. Rev. Cell Dev. Biol. 23, 549-577. 10.1146/annurev.cellbio.23.090506.123237 [DOI] [PubMed] [Google Scholar]
- Sillitoe, R. V., Gopal, N. and Joyner, A. L. (2009). Embryonic origins of ZebrinII parasagittal stripes and establishment of topographic Purkinje cell projections. Neuroscience 162, 574-588. 10.1016/j.neuroscience.2008.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva, T. P., Bekman, E. P., Fernandes, T. G., Vaz, S. H., Rodrigues, C. A. V., Diogo, M. M., Cabral, J. M. S. and Carmo-Fonseca, M. (2020a). Maturation of human pluripotent stem cell-derived cerebellar neurons in the absence of co-culture. Front. Bioeng. Biotechnol. 8, 70. 10.3389/fbioe.2020.00070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva, T. P., Fernandes, T. G., Nogueira, D. E. S., Rodrigues, C. A. V., Bekman, E. P., Hashimura, Y., Jung, S., Lee, B., Carmo-Fonseca, M. and Cabral, J. M. S. (2020b). Scalable generation of mature cerebellar organoids from human pluripotent stem cells and characterization by immunostaining. J. Vis. Exp. 10.3791/61143 [DOI] [PubMed] [Google Scholar]
- Solecki, D. J., Liu, X. L., Tomoda, T., Fang, Y. and Hatten, M. E. (2001). Activated Notch2 signaling inhibits differentiation of cerebellar granule neuron precursors by maintaining proliferation. Neuron 31, 557-568. 10.1016/s0896-6273(01)00395-6 [DOI] [PubMed] [Google Scholar]
- Sudarov, A. and Joyner, A. L. (2007). Cerebellum morphogenesis: the foliation pattern is orchestrated by multi-cellular anchoring centers. Neural Dev. 2, 26. 10.1186/1749-8104-2-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudarov, A., Turnbull, R. K., Kim, E. J., Lebel-Potter, M., Guillemot, F. and Joyner, A. L. (2011). Ascl1 genetics reveals insights into cerebellum local circuit assembly. J. Neurosci. 31, 11055-11069. 10.1523/JNEUROSCI.0479-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson, D. J., Tong, Y. and Goldowitz, D. (2005). Disruption of cerebellar granule cell development in the Pax6 mutant, Sey mouse. Brain Res. Dev. Brain Res. 160, 176-193. 10.1016/j.devbrainres.2005.09.005 [DOI] [PubMed] [Google Scholar]
- Szulc, K. U., Lerch, J. P., Nieman, B. J., Bartelle, B. B., Friedel, M., Suero-Abreu, G. A., Watson, C., Joyner, A. L. and Turnbull, D. H. (2015). 4D MEMRI atlas of neonatal FVB/N mouse brain development. Neuroimage 118, 49-62. 10.1016/j.neuroimage.2015.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda, N., Jain, R., LeBoeuf, M. R., Wang, Q., Lu, M. M. and Epstein, J. A. (2011). Interconversion between intestinal stem cell populations in distinct niches. Science 334, 1420-1424. 10.1126/science.1213214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallinen, T., Chung, J. Y., Biggins, J. S. and Mahadevan, L. (2014). Gyrification from constrained cortical expansion. Proc. Natl. Acad. Sci. USA 111, 12667-12672. 10.1073/pnas.1406015111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronche, F., Kellendonk, C., Kretz, O., Gass, P., Anlag, K., Orban, P. C., Bock, R., Klein, R. and Schütz, G. (1999). Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat. Genet. 23, 99-103. 10.1038/12703 [DOI] [PubMed] [Google Scholar]
- van der Heijden, M. E. and Zoghbi, H. Y. (2018). Loss of Atoh1 from neurons regulating hypoxic and hypercapnic chemoresponses causes neonatal respiratory failure in mice. eLife 7, e38455. 10.7554/eLife.38455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen, D. C. (2020). A 2020 view of tension-based cortical morphogenesis. Proc. Natl. Acad. Sci. USA 117, 32868-32879. 10.1073/pnas.2016830117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vladoiu, M. C., El-Hamamy, I., Donovan, L. K., Farooq, H., Holgado, B. L., Sundaravadanam, Y., Ramaswamy, V., Hendrikse, L. D., Kumar, S., Mack, S. C.et al. (2019). Childhood cerebellar tumours mirror conserved fetal transcriptional programs. Nature 572, 67-73. 10.1038/s41586-019-1158-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, V. Y., Rose, M. F. and Zoghbi, H. Y. (2005). Math1 expression redefines the rhombic lip derivatives and reveals novel lineages within the brainstem and cerebellum. Neuron 48, 31-43. 10.1016/j.neuron.2005.08.024 [DOI] [PubMed] [Google Scholar]
- Wang, S. S., Kloth, A. D. and Badura, A. (2014). The cerebellum, sensitive periods, and autism. Neuron 83, 518-532. 10.1016/j.neuron.2014.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, J. J. and Sillitoe, R. V. (2013). Postnatal development of cerebellar zones revealed by neurofilament heavy chain protein expression. Front. Neuroanat. 7, 9. 10.3389/fnana.2013.00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett, R. T., Bayin, N. S., Lee, A. S., Krishnamurthy, A., Wojcinski, A., Lao, Z., Stephen, D., Rosello-Diez, A., Dauber-Decker, K. L., Orvis, G. D.et al. (2019). Cerebellar nuclei excitatory neurons regulate developmental scaling of presynaptic Purkinje cell number and organ growth. eLife 8, e50617. 10.7554/eLife.50617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wizeman, J. W., Guo, Q., Wilion, E. M. and Li, J. Y. (2019). Specification of diverse cell types during early neurogenesis of the mouse cerebellum. eLife 8, e42388. 10.7554/eLife.42388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcinski, A., Lawton, A. K., Bayin, N. S., Lao, Z., Stephen, D. N. and Joyner, A. L. (2017). Cerebellar granule cell replenishment postinjury by adaptive reprogramming of Nestin+ progenitors. Nat. Neurosci. 20, 1361-1370. 10.1038/nn.4621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcinski, A., Morabito, M., Lawton, A. K., Stephen, D. N. and Joyner, A. L. (2019). Genetic deletion of genes in the cerebellar rhombic lip lineage can stimulate compensation through adaptive reprogramming of ventricular zone-derived progenitors. Neural Dev. 14, 4. 10.1186/s13064-019-0128-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada, M., Seto, Y., Taya, S., Owa, T., Inoue, Y. U., Inoue, T., Kawaguchi, Y., Nabeshima, Y. and Hoshino, M. (2014). Specification of spatial identities of cerebellar neuron progenitors by ptf1a and atoh1 for proper production of GABAergic and glutamatergic neurons. J. Neurosci. 34, 4786-4800. 10.1523/jneurosci.2722-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, H., Xie, X., Deng, M., Chen, X. and Gan, L. (2010). Generation and characterization of Atoh1-Cre knock-in mouse line. Genesis 48, 407-413. 10.1002/dvg.20633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung, J. and Goldowitz, D. (2017). Wls expression in the rhombic lip orchestrates the embryonic development of the mouse cerebellum. Neuroscience 354, 30-42. 10.1016/j.neuroscience.2017.04.020 [DOI] [PubMed] [Google Scholar]
- Yeung, J., Ha, T. J., Swanson, D. J. and Goldowitz, D. (2016). A novel and multivalent role of Pax6 in cerebellar development. J. Neurosci. 36, 9057-9069. 10.1523/JNEUROSCI.4385-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo, L., Theis, M., Alvarez-Maya, I., Brenner, M., Willecke, K. and Messing, A. (2001). hGFAP-cre transgenic mice for manipulation of glial and neuronal function in vivo. Genesis 31, 85-94. 10.1002/gene.10008 [DOI] [PubMed] [Google Scholar]
- Zong, H., Espinosa, J. S., Su, H. H., Muzumdar, M. D. and Luo, L. (2005). Mosaic analysis with double markers in mice. Cell 121, 479-492. 10.1016/j.cell.2005.02.012 [DOI] [PubMed] [Google Scholar]