Table 3.
Examples of representative imidazolone (12–17) and pyrazolone derivatives (18, 19) developed by Chiesi Farmaceutici S.p.A.

| ||||
|---|---|---|---|---|
| Compound | R1 | R2 | IC50 (nM)a | Reference |
| 12 |
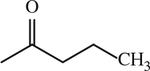
|
H | < 1 | [143] |
| 13 |
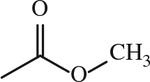
|
H | < 1 | [143] |
| 14 |
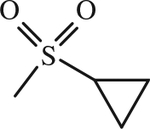
|
H | < 1 | [143] |
| 15 |
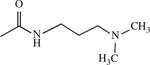
|
H | < 1 | [143] |
| 16 |
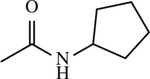
|
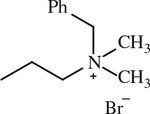
|
< 1 | [144] |
| 17 |
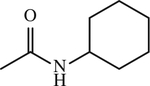
|
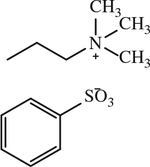
|
< 1 | [144] |
| 18 |
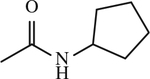
|
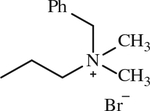
|
< 1 | [144] |
| 19 |
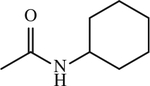
|
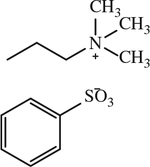
|
< 1 | [144] |
The results are means of two independent experiments, each performed in duplicate (HNE enzyme assay).
