Table 6.
Examples of condensed dihydropyrimidone derivatives developed by Boehringer Ingelheim GmbH.
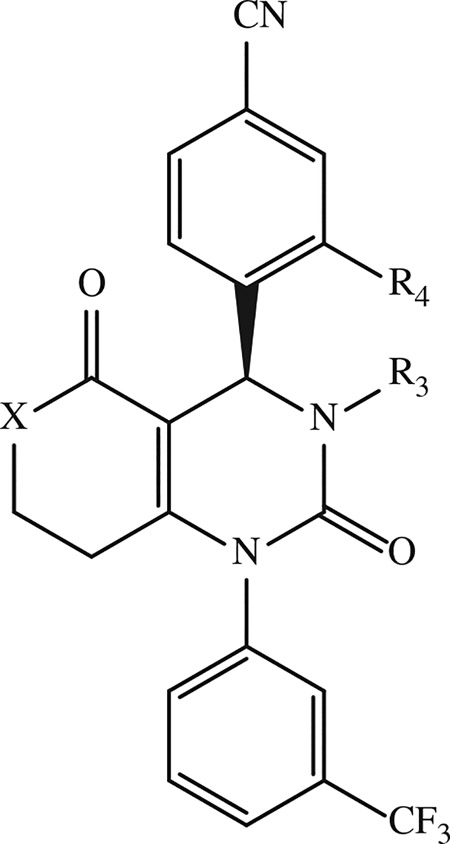
|
|||||
|---|---|---|---|---|---|
| Compound | R4 | R3 | X | IC50 (nM)a | Reference |
| 48 b | SO2CH3 | H | CH2 | 4.8 | [154] |
| 49 b | H |
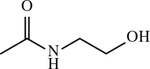
|
CH2 | < 1 | [154] |
| 50 b | H |
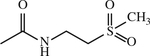
|
CH2 | < 1 | [154] |
| 51 b | SO2CH3 |

|
CH2 | < 1 | [154] |
| 52 b | SO2CH3 |
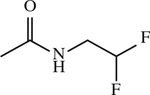
|
CH2 | < 1 | [154] |
| 53 b | SO2CH3 |
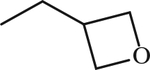
|
CH2 | 1 | [155] |
| 54 b | SO2CH3 | CH2CH2OH | CH2 | < 1 | [155] |
| 55 b | CONH2 | CH3 | CH2 | 1.6 | [152] |
| 56 c | H | H | NH | 3.1 | [156] |
| 57 c | H |

|
NH | 1.3 | [156] |
| 58 c | H |
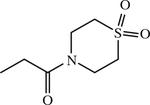
|
NH | < 1 | [156] |
| 59 c |

|
CH3 | NH | < 1 | [156] |
| 60 c | CONH2 | CH3 | N-CH3 | < 1 | [156] |
| 61 c | SO2CH3 | CH3 | NH | < 1 | [156] |
The results are means of two independent experiments, each performed in duplicate (HNE enzyme assay).
The absolute configuration (S) has been unambiguously assigned by X-ray structure analysis.
The absolute configuration (R) has been unambiguously assigned by X-ray structure analysis.
