Treatment options for cancer have evolved from that which is traditionally offered in either the hospital or clinic setting. Oral therapies are an emerging option that allow individuals to manage their condition from home. However, oral therapies bring unique challenges. Chief among them is suboptimal medication adherence. The World Health Organization defines adherence as “the extent to which a person’s behavior, taking medication, following a diet, and/or executing lifestyle changes, corresponds with agreed recommendations from a health care provider” (Sabaté, 2003, pg. 3). Suboptimal medication adherence has gained greater attention in medical and behavioral research due to its significant impact on the health of the population as well as its related burden on the health care system. Research has shown an estimated 125,000 deaths annually due to suboptimal medication adherence (Benjamin, 2012; Kleinsinger, 2018). Related health care costs are estimated as high as $100 billion annually (Kleinsinger, 2018). A review by Greer et al. (2016) showed adherence to oral therapies among cancer patients to run as low as 46%. Suboptimal adherence can lower treatment efficacy and the chance of achieving optimal control of disease (Spoelstra et al., 2013b). Therefore, it is critically important for nurses in oncology care to help identify effective ways to support optimal adherence to oral therapy.
According to the World Health Organization (Sabaté, 2003), there are 5 dimensions to adherence: health system-, patient-, social/economic-, condition-, and therapy- related factors. A review by Goh et al. (2017) supports the concept of adherence as a multifactorial phenomenon. Their review, which focused on the pediatric oncology population, found patient/caregiver-related factors include patient’s personality, demographics, disease and treatment perceptions, and social support. Therapy-related factors include side effects, length and complexity of treatment, and route of administration. Condition-related factors include disease prognosis. Health system-related factors include health literacy, access to healthcare, patient: provider relations, and perception of hospital care. Social/economic factors include financial difficulties, transportation issues, and family dynamics. Similar findings were seen in the adult oncology population (Irwin & Johnson, 2015).
Forgetfulness, considered a patient-related factor, is among the leading factors contributing to suboptimal medication adherence (Goh et al., 2017). Reminder notifications delivered via text message and smart phone applications are among the mobile health interventions that are growing in popularity and have proven efficacy at improving adherence in various chronic conditions (Hammonds et al., 2015; Weisman et al., 2018). However, there is limited research available on the effectiveness of such interventions at improving adherence to medications among oncology patients. This literature review aims to summarize the extant literature on medication adherence outcomes for mobile health interventions used in the oncology population.
Mobile Health Interventions
Mobile health (mHealth) interventions use mobile technology (i.e., smartphones) to enhance healthcare delivery. According to the Pew Research Center (2019), 96% of Americans own mobile phones, 81% of which are smart phones. The wide accessibility and use of smartphones in the United States support mobile technology as a tool to foster healthy behaviors. The use of mHealth interventions for medication adherence has been studied in a variety of health care specialties, and have demonstrated statistically significant improvements in medication adherence and, in some cases, clinical outcomes among individuals with hyperlipidemia, myocardial infarction, attention-deficit/hyperactivity disorder (ADHD), the human immunodeficiency virus (HIV), and hypertension (Fang & Li, 2016; Johnston et al., 2016; Márquez Contreras et al., 2019; Perera et al., 2014; Weisman et al., 2018).
Skrabal Ross et al. (2018) completed a scoping review of the existing literature on mHealth interventions for oral chemotherapy adherence. Interventions were mainly comprised of smartphone applications and short message service (SMS) reminders. Strategies included symptom reporting, symptom management, and medication dosing reminders. The review found the interventions useful and feasible in the oncology setting. However, the authors identified a need for further research on the impact of mobile interventions on adherence outcomes in oncology (Skrabal Ross et al., 2018). The research question for this review is: are mHealth interventions an effective approach to improve medication adherence in the oncology setting?
Method
A database search of PubMed®, MEDLINE®, and the Cumulative Index to Nursing and Allied Health Literature (CINAHL®) was conducted using a combination of the following search terms: text messaging or texting or sms messaging, mhealth or mobile health or mobile application, cellphones or smartphones or mobile phones, smartphone, medication adherence or compliance or medication nonadherence or medication noncompliance, medication self-management, oncology or cancer. Studies were included if they were primary research, focused on the cancer population, utilized mobile technology as the medication adherence intervention, quantitatively measured adherence outcomes, and were English language.
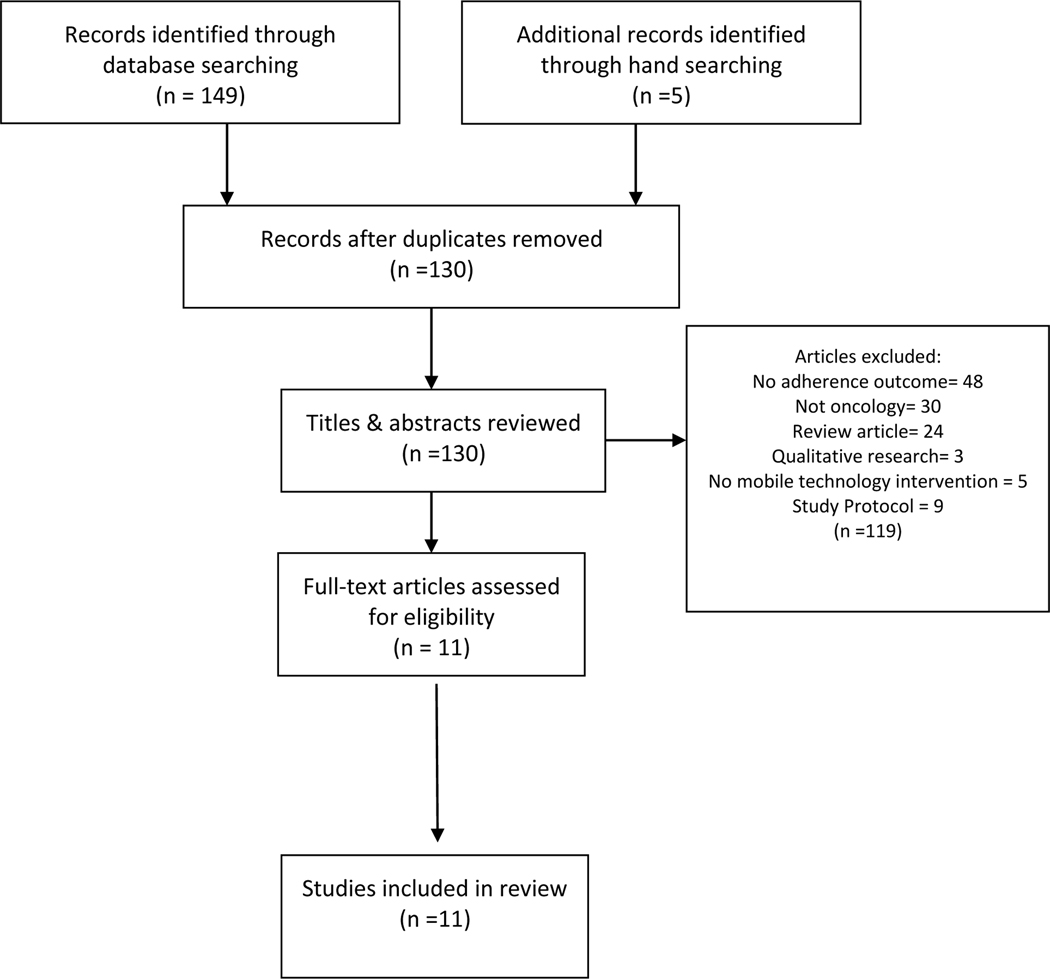
Two searches were completed between November 2019 and December 2020. One hundred and forty-nine titles resulted from this search. After duplicates were removed, 130 titles and abstracts were reviewed, including five studies identified via hand-searching of selected reference lists. Eleven peer-reviewed studies were found eligible and included in this review (see Figure 1).
Figure 1.
Search Strategy
Results
The eleven studies included in this review cover the time-period between 2013–2020. The interventions studied include automated phone calls, text message reminders, and smartphone applications. All but one study was conducted in the United States; Kim et al. (2018) was performed in South Korea. Adherence was primarily measured using subjective methods such as self-report surveys, medication possession ratio (MPR), relative dose intensity (RDI), and electronic pill caps. Krok-Schoen et al. (2019) also measured hormonal levels pre- and post-study and Hershman et al. (2020) measured urine aromatase inhibitor assay levels as objective adherence measures. Reliability data for study instruments were described by Kim et al. (2018) who reported Cronbach alpha of 0.71 for the Korean Medication Adherence Rating Scale (K-MARS), and Spoelstra et al. (2015) reported Cronbach alpha 0.66 for the Medication Adherence Rating Scale (MARS).
Automated phone calls
Two studies investigated the effects of automated voice response call reminders on medication adherence and symptom management outcomes. One study was a 3-group exploratory pilot study (Spoelstra et al., 2013a) and the other a double arm randomized controlled trial (Sikorskii et al., 2018). Participants in Spoelstra et al. (2013a) included individuals with breast (n = 39), colorectal (n = 11), lung (n = 30), and ‘other’ oncological diseases (n = 39). Oral anti-cancer treatment regimens in Spoelstra et al. (2013a) were described as ‘complex’ (n = 55) and ‘noncomplex’ (n = 64). Participants in Sikorskii et al. (2018) included individuals with a variety of solid tumor and hematologic malignancies (see Table 1). Oral treatments in Sikorskii et al. (2018) included cytotoxic agents (n = 95), kinase inhibitors (n = 127), sex hormone inhibitors (n = 27), and ‘other’ (n = 23). The mean age in Spoelstra et al. (2013a) was 59.6, and in Sikorskii et al. (2018) the mean age was 61. Study durations ranged from 10–12 weeks.
Table 1.
Literature Review
| Study | Design | Sample Size/ Diagnosis (N) | Age (mean) | Treatment | Intervention | Adherence Measure | Outcome |
|---|---|---|---|---|---|---|---|
| Spoelstra et al., 2013 | Three-arm pilot exploratory study | N= 91 Breast (39), Colon/rectal (11), Lung (30), Other (39) | 59.6 | Oral chemotherapy | Randomization to either: 1) AVR/SMT 2) AVR/SMT + nurse strategies to manage symptoms and improve adherence 3) AVR/SMT + nurse strategies to improve adherence. | Self-report | No significant difference in adherence between intervention arms. |
| Spoelstra et al., 2015 | Double-arm, longitudinal randomized controlled trial | N=80 Breast (15), Prostate (9), Lung (8), Colon (7), Multiple Myeloma (6), Renal (4), Leukemia (4), Esophageal (2), Liver (1), Brain (1), Kidney (1), Pancreatic (1), Rectal (1) Melanoma (1), Other (15) | 58.6 | Oral chemotherapy | Text message +/− automated voice recording reminders | Self-report and RDI | RDI was greater in the intervention group but not at statistically significant level. Adherence was higher in the intervention group for several weeks throughout the study but was not consistent. |
| Spoelstra et al., 2016 | Multisite, double-arm, longitudinal, randomized controlled trial | N=75 Breast (19) Other (56) | 60 | Oral chemotherapy | Text message reminders vs. usual care | Self-report and pill counts | Control group had higher adherence rates early on in the study, whereas the experimental groups adherence was higher in the long-term. |
| Graetz et al., 2018 | Pilot randomized controlled trial | N=48 All breast cancer | 59.9 | Aromatase inhibitors | Smartphone app +/− text and/or email reminders | Self-report | APP+ reminder group had greater adherence at a statistically significant level. |
| Kim et al., 2018 | Randomized controlled trial | N=76 All breast cancer | 50.9 | Oral chemotherapy | Web-based game app | Self-report | Mean adherence scores were significantly higher in the intervention group. |
| Sikorskii et al., 2018 | Two-arm, randomized controlled trial | N= 229 Breast (57), colorectal (41), GI (17), leukemia (16), liver (12), lung (10), lymphoma (3), melanoma (8), myeloma (7), pancreatic (27), prostate (26), renal (24), sarcoma (15), brain (2), esophageal (3), other (4) | 61 | Oral chemotherapy | Automated telephone medication reminders & symptom assessment with weekly symptom management intervention as needed. | RDI | No observed difference in RDI post intervention. |
| Linder et al., 2019 | mixed-method single-group intervention longitudinal design | N=23 Leukemia (8), lymphoma (4), sarcoma (4) brain (4), other (3) | 20.2 | Oral chemotherapy, antibiotics, and other cancer-related supportive medication | Smartphone app | Electronic pill caps | No change in adherence pre-to-post intervention. |
| Krok-Schoen et al., 2019 | Cross-sectional pilot study | N=27 All breast cancer | 59.7 | Adjuvant hormonal therapy | Daily text messages & weekly smartphone app surveys on adherence and symptoms. | Self-report and hormonal biomarkers | Self-reported adherence significantly improved from baseline to end-of-study. Hormone levels significantly declined by the exit interview. |
| Greer et al., 2020 | 1:1 parallel assignment, randomized controlled trial | N = 181 Hematologic (60), NSCLC (33), breast (26), high-grade glioma (26), sarcoma (12), GI (8), GU (7), melanoma (7), non-GIST (2) | 53 | Targeted therapy and chemotherapy | Smartphone app | Electronic pill caps and self-report survey | No differences noted in adherence (pill count or self-report). |
| Hershman et al., 2020 | Multicenter randomized trial | N = 702 All breast cancer | 60.9 | Aromatase Inhibitors | Twice weekly educational text messages | AI urine assay | No difference in rates of adherence failure between both arms of the study. |
| Bhatia et al., 2020 | Unblinded, parallel-group, randomized trial | N = 444 All acute lymphoblastic leukemia | 8.1 | Oral chemotherapy | Daily text-message reminder | Electronic pill caps | No significant difference found between groups in proportion achieving adherence rates 95% or higher. |
AI: Aromatase Inhibitor
AVR: automated voice recording
HU: Hydroxyurea
MPR: medication possession ratio
RDI: relative dose intensity
SMT: symptom management toolkit
The interventions consisted of routine automated telephone calls that assessed medication adherence (Spoelstra et al., 2013a) or reminded patients of their scheduled doses (Sikorskii et al., 2018). Symptoms were also assessed and managed via automated calls (Sikorskii et al., 2018; Spoelstra et al., 2013a). Calls for adherence were made either daily (Sikorskii et al., 2018) or weekly (Spoelstra et al., 2013a). In Spoelstra et al. (2013a) three arms of the study included automated calls alone or automated calls plus additional calls from a nurse who provided guidance on medication adherence and/or symptom management, depending on patient feedback during automated calls. Spoelstra et al. (2013a) measured adherence by self-report, while Sikorskii et al. (2018) used RDI.
There were no significant differences in adherence outcomes between treatment arms in either study. However, Spoelstra et al. (2013a) noted a trend toward greater adherence rates among study groups randomized to receive additional nursing phone calls albeit not at a statistically significant level; calls for symptom management and adherence (P = 0.11), calls for adherence only (P = .54).
Text message reminders
Five studies investigated the use of text message reminders. Designs included four randomized trials and one repeated measures study. Sample sizes ranged from 27–702, and subject’s age ranged from 8-to-60 years old (see Table 1). Diagnoses included breast cancer (n=2), acute lymphoblastic leukemia (ALL; n=1), and two studies that included a variety of cancer diagnoses (Spoelstra et al., 2015; Sandra L. Spoelstra et al., 2016). Participants received treatment with a variety of oral hormonal and nonhormonal anticancer agents. Study durations ranged from 10 weeks to 2 years.
Interventions included 3 studies focused on daily text message reminders of medication dosing (Krok-Schoen et al., 2019; Spoelstra et al., 2015; S. L. Spoelstra et al., 2016). Participants Spoelstra et al. (2015) and Spoelstra et al. (2016) received routine symptom assessment via automated voice response calls; Krok-Schoen et al. (2019) offered assessments via smartphone app, which could be forwarded to the patient’s physician. Bhatia et al. (2020) randomized pediatric ALL participants in their study to an education program plus daily text-message reminders or the education program alone. Intervention group participants and their parents received daily text-message reminders from their physicians, delivered via an interactive web-based application, to prompt daily dosing of oral mercaptopurine (Bhatia et al., 2020). The education program consisted of video vignettes focused on ALL and mercaptopurine treatment, barriers to adherence, and ways to address those barriers (Bhatia et al., 2020). Patients and parents viewed the educational video on study day 29, during a scheduled clinic visit; daily text message reminders began on day 29, for those in the intervention group, and were renewed by the physician every 28 days for 16 weeks (Bhatia et al., 2020). Hershman et al. (2020) studied twice weekly educational text messages that focused on overcoming barriers to adherence, cues to action, statements related to treatment efficacy, reinforcement of physicians’ recommendations to take medication, and providing words of support.
No statistically significant changes in adherence were found in either Spoelstra et al. (2015) or Spoelstra et al. (2016). Krok-Schoen et al. (2019) found significant improvement in self-reported adherence from baseline to end-of-study (p= 0.015) as well as a significant decline in hormone levels (p<.001) by the exit interview. While Hershman et al. (2020) found no difference in rate of adherence failure between both arms of their study, a statistically significant difference in intervention effect by race (P = 0.04) was seen. However, the specifics of how outcomes differed by race were not provided (Hershman, 2020). There also appeared to be a beneficial effect on participants who were older (>65), treated at a teaching hospital, had higher copayments, and lacking private insurance (Hershman et al., 2020). Bhatia et al. (2020) found no significant difference between groups on proportion achieving mercaptopurine adherence rates of 95% or higher, after adjusting for baseline adherence, time in study, and paternal education (OR 1.33, 95% CI 1.0–2.0; P=.08). However, participants with ≥ 95% adherence at baseline who received the education program alone showed greater declines in adherence rates than those who received the intervention (Bhatia et al., 2020). Interestingly, among participants 12 years and older with lower baseline adherence, adherence rates were significantly higher among the group that received text message reminders (83.4% vs 74.6%; P = .008) (Bhatia et al., 2020).
Smartphone Applications
Smartphone applications were the subject of four studies. Designs included three randomized trials and a mixed-method single group intervention longitudinal trial (see Table 1). Sample sizes ranged from 23–181. Subjects age ranged from 20–60 years old. Diagnoses included a variety of solid and hematologic malignancies, and participants were receiving a variety of treatments including aromatase inhibitors, chemotherapy, and antibiotics (see Table 1). Study durations ranged from 3–12 weeks.
Graetz et al. (2018a) used an interactive web-based application that allowed subjects to record their symptoms and adherence. The app also enabled direct communication between subjects and their care providers and interfaced with the patient’s electronic medical record (EMR). Subjects in Graetz et al. (2018a) all received the app; randomization occurred to either app alone or with text message reminders to use the app. Linder et al. (2019) utilized a smartphone medication reminder app that delivered routine dosing reminders. Kim et al. (2018) studied an educational mobile gaming application to improve treatment adherence, symptom management, and psychological distress among breast cancer patients on chemotherapy. Participants were randomized to either the ILOVEBREAST educational web-based gaming app, which included personal avatars, social networking, information on symptom management, and psychological support, or a 26-page educational brochure offering strategies for coping with chemotherapy side effects (Kim et al., 2018). Participants in each arm were instructed to either utilize the app or read the brochure for >30 minutes/day, 3 times per week (Kim et al., 2018). Greer et al. (2020) randomized their participants to use of a smartphone application they developed that included a personalized medication dosing schedule with optional reminders, adherence and symptom assessment modules, educational resources, and Fitbit integration or usual clinical care.
Kim et al. (2018) found significantly higher adherence scores in the group using the ILOVEBREAST app at end-of-study (7.6 vs. 6.5; p<.001). Graetz et al. (2018a) found statistically higher levels of adherence in the group that received text message reminders to use the smartphone app (p= <.05). However, the app’s actual effect on adherence is unclear as all participants had access to it (Graetz et al., 2018a). Overall, Greer et al. (2020) found no difference in adherence outcomes between study groups. However, participants in the mobile app group who had baseline adherence problems or higher anxiety levels at baseline showed higher adherence rates compared with standard of care; 86.23% vs. 63.94% (P = .034) and 85.45% vs. 69.39% (P = .044) respectively. Additionally, participants who spent more time using the app were found to take greater proportions of their medications (r = .29; P = .022) (Greer et al., 2020). Linder et al. (2019) found no appreciable change in adherence pre-and-post intervention.
Patient Feedback on Interventions
Among the studies that elicited participants’ satisfaction, interventions were overall rated satisfactory and helpful at improving medication adherence (Graetz et al., 2018a; Kim et al., 2018; Krok-Schoen et al., 2019; Linder et al., 2019; Spoelstra et al., 2015). However, Spoelstra et al., notes considerable attrition post-intervention within the intervention arms of each of their studies. Individuals in each study were either lost to follow-up or withdrew from the study; 24% (2013a), 15% (2015), and 14% (2016).
Discussion
Eleven articles were identified that the measured medication adherence outcomes of mHealth interventions used exclusively among patients with cancer. Similar to Burhenn and Smudde (2015), this review finds that interventions thus far have largely included automated voice response, text messaging, and smartphone applications. While patients were overall satisfied with their use of these interventions and found them helpful, adherence outcomes were mixed.
The adherence findings may in part be due to the variety of methods, samples, and interventions included in this review. Similar outcomes were noted by Mathes et al. (2014) in their systematic review of adherence interventions for oral anticancer agents and Anglada-Martinez et al. (2015) in their systematic review of mHealth in chronic disease, HIV, and healthy populations. A meta-analysis of electronic reminders in chronic disease by Tao et al. (2015) found overall improvement in adherence among their diverse group of studies but pooled effect size was small. Additionally, several articles in this review were small pilot studies aimed at establishing proof of concept and therefore not sufficiently powered to detect intervention effects. Adherence measures were also largely subjective which, though a cost effective and common method used in adherence research, has been shown to vary significantly from findings obtained with objective measures (Atkinson et al., 2016). Lastly, the lack of a standard adherence definition made comparison among studies a challenge. This limitation has been highlighted in previous reviews on medication adherence in oncology (Greer et al., 2016; Mathes et al., 2014; Morrison et al., 2017).
The interventions in this review mainly functioned as cue reminders for medication dosing, though several also offered education and symptom management support. Interestingly, interventions associated with statistical improvements in adherence included interactive components that provided for feedback and communication with medical providers, educational tools, and/or social networking (Graetz et al., 2018b; Kim et al., 2018; Krok-Schoen et al., 2019). A similar trend exists among studies on adherence interventions in HIV (Perera et al., 2014) and hypertension (Márquez Contreras et al., 2019). Given the multifactorial nature of medication adherence, of which forgetfulness is only one of many determinants, it stands to reason that studies employing multidimensional interventions most effectively demonstrated positive outcomes.
The importance of tailoring mobile health adherence interventions to patients’ specific needs was highlighted in this review. Sikorskii et al. (2018) failed to demonstrate improvements in adherence among their sample, who were highly adherent at baseline, while Bhatia et al. (2020) and Greer et al. (2020) showed statistically significant improvements in adherence measures specifically among their participants with lower baseline adherence. Kjos et al. (2019) found a similar trend in their study on a medication adherence app for type 2 diabetes.
While various cancer types were included in this review, nearly half of the studies focused on individuals with breast cancer. A notable exemption was the use of oral immunosuppressants in the hematopoietic stem cell transplant (HSCT) population. Suboptimal adherence rates among HSCT patients have consistently been found to run over 50% (Gresch et al., 2017; Lehrer et al., 2018). As CorrÊA et al. (2016) note, suboptimal adherence with immunosuppressant medications can result in significant morbidity due to graft-vs.-host disease; this highlights the need for more research on adherence interventions in the HSCT population. The samples included in this study were also overwhelmingly older, white, well educated, and middle to upper economic status. Graetz et al. (2018a) had a more diverse sample with 25% of participants being racial minorities, 28% with low health literacy, and nearly 40% with incomes of ≤ 150% of the federal poverty level. Similarly, Bhatia et al. (2020) included 40% Hispanic, 9% African American, and 12% Asian or mixed raced participants in their sample of whom roughly 26% earned <$20,000/year. Greater diversity is needed in adherence research, particularly concerning mobile interventions, when considering the potential impacts of technological literacy and access, healthcare access and health literacy on outcomes.
Nursing Implications
According to the World Health Organization (Sabaté, 2003), nursing strategies to improve adherence include “suggesting cues and reminders such as detailed schedules, integrating medication times with daily habits, using medication boxes and timers, alarms, beepers, etc.,” (pg. 158). Mobile health interventions align with these strategies and provide a means by which oncology nurses may help to improve patient adherence to medications. The use of mobile health interventions for adherence need not require the purchase or subscription to a smartphone application or service. Patients who are prone to forgetfulness can be instructed to set timers on their smartphones to alarm them at the designated time of dosing (Burhenn & Smudde, 2015). An important safety consideration is the need to adjust reminder alarms for treatment holidays, drug discontinuation, or other periods where treatment will not be continued. Additionally, Hershman et al. (2020) noted that increased text messaging and electronic alerts can create alert fatigue, thus limiting the impact of the interventions. Setting an alert frequency that is effective and does not overburden the patient is critical.
The findings of this review demonstrate the importance of targeting mobile health interventions to the individual needs of each patient. The Washburn-Barriers to Medication Adherence Screening Instrument (Washburn & Thompson, 2020) is one instrument under development that may offer nurses a means of systematic assessment for patients at greatest risk for adherence challenges and may benefit most from mobile health interventions.
The use of mobile health interventions alone may not be sufficient for demonstrable improvement in adherence outcomes. Across the studies in this review, clinician interaction with patients augmented the effects of those interventions that included this additional support. These findings suggest that clinician support (i.e., nursing assessments, symptom management) support patients’ ability to adhere to their medication regimens. The ability to social network and receive feedback on adherence are also significant components of mobile health intervention success, which nurses are well-positioned to provide.
Conclusion
This review finds mHealth interventions are an acceptable approach that may improve adherence outcomes for patients with cancer. The findings above are in concordance with extant research findings on mHealth in other chronic diseases. This review’s findings support the use of mobile health interventions that are tailored to patient’s needs and multidimensional in their approach. Implications for further research include the need for large, powered studies, greater standardization of adherence definitions, greater use of biomarkers and other objective adherence measures, and greater diversity among research participants.
Figure 2.
Medication Adherence Calculations
Based on information from Crowe (2015) and Engle et al. (2018).
Footnotes
Conflicts of interest: None
Reference:
- Anglada-Martinez H, Riu-Viladoms G, Martin-Conde M, Rovira-Illamola M, Sotoca-Momblona JM, & Codina-Jane C.(2015). Does mHealth increase adherence to medication? Results of a systematic review. International Journal of Clinical Practice, 69(1), 9–32. 10.1111/ijcp.12582 [DOI] [PubMed] [Google Scholar]
- Atkinson TM, Rodríguez VM, Gordon M, Avildsen IK, Emanu JC, Jewell ST, & Anselmi KA (2016). The Association Between Patient-Reported and Objective Oral Anticancer Medication Adherence Measures: A Systematic Review. Oncology Nursing Forum, 43(5), 576–582. 10.1188/16.ONF.576-582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin RM (2012). Medication Adherence: Helping Patients Take Their Medicines as Directed. Public Health Reports, 127(1), 2–3. 10.1177/003335491212700102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia S, Hageman L, Chen Y, Wong FL, McQuaid EL, Duncan C, Mascarenhas L, Freyer D, Mba N, Aristizabal P, Walterhouse D, Lew G, Kempert PH, Russell TB, McNall-Knapp RY, Jacobs S, Dang H, Raetz E, Relling MV, & Landier W.(2020). Effect of a Daily Text Messaging and Directly Supervised Therapy Intervention on Oral Mercaptopurine Adherence in Children With Acute Lymphoblastic Leukemia: A Randomized Clinical Trial. JAMA Netw Open, 3(8), e2014205. 10.1001/jamanetworkopen.2020.14205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burhenn PS, & Smudde J.(2015). Using Tools and Technology to Promote Education and Adherence to Oral Agents for Cancer. Clinical Journal of Oncology Nursing, 19, 53–59. 10.1188/15.S1.CJON.53-59 [DOI] [PubMed] [Google Scholar]
- CorrÊA PM, Zuckermann J, Fischer GB, & Castro MS (2016). Immunosuppressive serum levels in allogeneic hematopoietic stem cell transplantation: pharmaceutical care contribution. Pharmacy Practice (1886–3655), 14(2), 1–5. 10.18549/PharmPract.2016.02.683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engle JA, Traynor AM, Campbell TC, Wisinski KB, Loconte N, Liu G, Wilding G, & Kolesar JM (2018). Assessment of adherence and relative dose intensity with oral chemotherapy in oncology clinical trials at an academic medical center. Journal of Oncology Pharmacy Practice, 24(5), 348–353. 10.1177/1078155217704989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang R, & Li X.(2016). Electronic messaging support service programs improve adherence to lipid-lowering therapy among outpatients with coronary artery disease: an exploratory randomised control study. Journal of Clinical Nursing (John Wiley & Sons, Inc.), 25(5–6), 664–671. 10.1111/jocn.12988 [DOI] [PubMed] [Google Scholar]
- Goh XTW, Tan YB, Thirumoorthy T, & Kwan YH (2017). A systematic review of factors that influence treatment adherence in paediatric oncology patients. Journal of Clinical Pharmacy & Therapeutics, 42(1), 1–7. 10.1111/jcpt.12441 [DOI] [PubMed] [Google Scholar]
- Graetz I, McKillop CN, Stepanski E, Vidal GA, Anderson JN, & Schwartzberg LS (2018b). Use of a web-based app to improve breast cancer symptom management and adherence for aromatase inhibitors: a randomized controlled feasibility trial. Journal Of Cancer Survivorship: Research And Practice, 12(4), 431–440. 10.1007/s11764-018-0682-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer JA, Amoyal N, Nisotel L, Fishbein JN, MacDonald J, Stagl J, Lennes I, Temel JS, Safren SA, & Pirl WF (2016). A Systematic Review of Adherence to Oral Antineoplastic Therapies. Oncologist, 21(3), 354–376. 10.1634/theoncologist.2015-0405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer JA, Jacobs JM, Pensak N, Nisotel LE, Fishbein JN, MacDonald JJ, Ream ME, Walsh EA, Buzaglo J, Muzikansky A, Lennes IT, Safren SA, Pirl WF, & Temel JS (2020). Randomized Trial of a Smartphone Mobile App to Improve Symptoms and Adherence to Oral Therapy for Cancer. J Natl Compr Canc Netw, 18(2), 133–141. 10.6004/jnccn.2019.7354 [DOI] [PubMed] [Google Scholar]
- Gresch B, Kirsch M, Fierz K, Halter JP, Nair G, Denhaerynck K, & De Geest S.(2017). Medication nonadherence to immunosuppressants after adult allogeneic haematopoietic stem cell transplantation: a multicentre cross-sectional study. Bone Marrow Transplantation, 52(2), 304–306. 10.1038/bmt.2016.262 [DOI] [PubMed] [Google Scholar]
- Hammonds T, Rickert K, Goldstein C, Gathright E, Gilmore S, Derflinger B, Bennett B, Sterns A, Drew BL, & Hughes JW (2015). Adherence to Antidepressant Medications: A Randomized Controlled Trial of Medication Reminding in College Students. Journal of American College Health, 63(3), 204–208. 10.1080/07448481.2014.975716 [DOI] [PubMed] [Google Scholar]
- Hershman DL, Unger JM, Hillyer GC, Moseley A, Arnold KB, Dakhil SR, Esparaz BT, Kuan MC, Graham Ii ML, Lackowski DM, Edenfield WJ, Dayao ZR, Henry NL, Gralow JR, Ramsey SD, Neugut AI, & Graham ML 2nd, (2020). Randomized Trial of Text Messaging to Reduce Early Discontinuation of Adjuvant Aromatase Inhibitor Therapy in Women With Early-Stage Breast Cancer: SWOG S1105. Journal of Clinical Oncology, 38(19), 2122–2129. 10.1200/JCO.19.02699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin M, & Johnson LA (2015). Factors Influencing Oral Adherence: Qualitative Metasummary and Triangulation With Quantitative Evidence. Clinical Journal of Oncology Nursing, 19, 6–30. 10.1188/15.S1.CJON.6-30 [DOI] [PubMed] [Google Scholar]
- Johnston N, Bodegard J, Jerström S, Åkesson J, Brorsson H, Alfredsson J, Albertsson PA, Karlsson J-E, & Varenhorst C.(2016). Effects of interactive patient smartphone support app on drug adherence and lifestyle changes in myocardial infarction patients: A randomized study. American Heart Journal, 178, 85–94. 10.1016/j.ahj.2016.05.005 [DOI] [PubMed] [Google Scholar]
- Kim HJ, Kim SM, Shin H, Jang J-S, Kim YI, & Han DH (2018). A Mobile Game for Patients With Breast Cancer for Chemotherapy Self-Management and Quality-of-Life Improvement: Randomized Controlled Trial. Journal Of Medical Internet Research, 20(10), e273–e273. 10.2196/jmir.9559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjos AL, Vaughan AG, & Bhargava A.(2019). Impact of a mobile app on medication adherence and adherence-related beliefs in patients with type 2 diabetes. Journal of the American Pharmacists Association: JAPhA, 59, S44–S51.e43. 10.1016/j.japh.2018.12.012 [DOI] [PubMed] [Google Scholar]
- Kleinsinger F.(2018). The Unmet Challenge of Medication Nonadherence. The Permanente Journal. 10.7812/tpp/18-033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krok-Schoen JL, Naughton MJ, Young GS, Moon J, Poi M, Melin SA, Wood ME, Hopkins JO, Paskett ED, & Post DM (2019). Increasing Adherence to Adjuvant Hormone Therapy Among Patients With Breast Cancer: A Smart Phone App-Based Pilot Study. Cancer Control: Journal Of The Moffitt Cancer Center, 26(1), 1073274819883287-1073274819883287. 10.1177/1073274819883287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer J, Brissot E, Ruggeri A, Dulery R, Vekhoff A, Battipaglia G, Giannotti F, Fernandez C, Mohty M, & Antignac M.(2018). Medication adherence among allogeneic hematopoietic stem cell transplant recipients: a pilot single-center study. Bone Marrow Transplantation, 53(2), 231–233. 10.1038/bmt.2017.233 [DOI] [PubMed] [Google Scholar]
- Linder LA, Wu YP, Macpherson CF, Fowler B, Wilson A, Jo Y, Jung S-H, Parsons B, & Johnson R.(2019). Oral Medication Adherence Among Adolescents and Young Adults with Cancer Before and Following Use of a Smartphone-Based Medication Reminder App. Journal of Adolescent & Young Adult Oncology, 8(2), 122–130. 10.1089/jayao.2018.0072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Márquez Contreras E, Márquez Rivero S, Rodríguez García E, López-García-Ramos L, Carlos Pastoriza Vilas J, Baldonedo Suárez A, Gracia Diez C, Gil Guillén V, Martell Claros N, Compliance Group of Spanish Society of, H., & Pastoriza Vilas JC (2019). Specific hypertension smartphone app to improve medication adherence in hypertension: a cluster-randomized trial. Current Medical Research & Opinion, 35(1), 167–173. 10.1080/03007995.2018.1549026 [DOI] [PubMed] [Google Scholar]
- Mathes T, Antoine S-L, Pieper D, & Eikermann M.(2014). Adherence enhancing interventions for oral anticancer agents: a systematic review. Cancer Treatment Reviews, 40(1), 102–108. 10.1016/j.ctrv.2013.07.004 [DOI] [PubMed] [Google Scholar]
- Morrison CF, Martsolf DM, Wehrkamp N, Tehan R, & Pai ALH (2017). Medication Adherence in Hematopoietic Stem Cell Transplantation: A Review of the Literature. Biology of Blood and Marrow Transplantation, 23(4), 562–568. 10.1016/j.bbmt.2017.01.008 [DOI] [PubMed] [Google Scholar]
- Perera AI, Thomas MG, Moore JO, Faasse K, & Petrie KJ (2014). Effect of a Smartphone Application Incorporating Personalized Health-Related Imagery on Adherence to Antiretroviral Therapy: A Randomized Clinical Trial. AIDS Patient Care & STDs, 28(11), 579–586. 10.1089/apc.2014.0156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pew Research Center. (2019, June 12). Mobile Phone Fact Sheet. Internet & Technology. https://www.pewresearch.org/internet/fact-sheet/mobile/
- Sabaté E.& World Health Organization. (2003). Adherence to long-term therapies: Evidence for action. Geneva: World Health Organization [Google Scholar]
- Sikorskii A, Given CW, Given BA, Vachon E, Krauss JC, Rosenzweig M, McCorkle R, Champion VL, Banik A, & Majumder A.(2018). An Automated Intervention Did Not Improve Adherence to Oral Oncolytic Agents While Managing Symptoms: Results From a Two-Arm Randomized Controlled Trial. Journal of Pain & Symptom Management, 56(5), 727–735. 10.1016/j.jpainsymman.2018.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrabal Ross X, Gunn KM, Patterson P, & Olver I.(2018). Mobile-Based Oral Chemotherapy Adherence–Enhancing Interventions: Scoping Review. JMIR mHealth and uHealth, 6(12), e11724. 10.2196/11724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoelstra SL, Given BA, Given CW, Grant M, Sikorskii A, You M, & Decker V.(2013a). An intervention to improve adherence and management of symptoms for patients prescribed oral chemotherapy agents: an exploratory study. Cancer Nursing, 36(1), 18–28. 10.1097/NCC.0b013e3182551587 [DOI] [PubMed] [Google Scholar]
- Spoelstra SL, Given BA, Given CW, Grant M, Sikorskii A, You M, & Decker V.(2013b). Issues Related to Overadherence to Oral Chemotherapy or Targeted Agents. Clinical Journal of Oncology Nursing, 17(6), 604–609. 10.1188/13.CJON.17-06AP [DOI] [PubMed] [Google Scholar]
- Spoelstra SL, Given CW, Sikorskii A, Coursaris CK, Majumder A, DeKoekkoek T, Schueller M, & Given BA (2015). Feasibility of a Text Messaging Intervention to Promote Self-Management for Patients Prescribed Oral Anticancer Agents. Oncology Nursing Forum, 42(6), 647–657. 10.1188/15.ONF.647-657 [DOI] [PubMed] [Google Scholar]
- Spoelstra SL, Given CW, Sikorskii A, Coursaris CK, Majumder A, DeKoekkoek T, Schueller M, & Given BA (2016). Proof of Concept of a Mobile Health Short Message Service Text Message Intervention That Promotes Adherence to Oral Anticancer Agent Medications: A Randomized Controlled Trial. Telemedicine Journal And E-Health: The Official Journal Of The American Telemedicine Association, 22(6), 497–506. 10.1089/tmj.2015.0126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao D, Xie L, Wang T, & Wang T.(2015). A meta-analysis of the use of electronic reminders for patient adherence to medication in chronic disease care. Journal of Telemedicine & Telecare, 21(1), 3–13. 10.1177/1357633X14541041 [DOI] [PubMed] [Google Scholar]
- Washburn DJ, & Thompson K.(2020). Medication Adherence Barriers: Development and Retrospective Pilot Test of an Evidence-Based Screening Instrument. Clin J Oncol Nurs, 24(2), E13–E20. 10.1188/20.CJON.E13-E20 [DOI] [PubMed] [Google Scholar]
- Weisman O, Schonherz Y, Harel T, Efron M, Elazar M, & Gothelf D.(2018). Testing the Efficacy of a Smartphone Application in Improving Medication Adherence, Among Children with ADHD. Israel Journal of Psychiatry & Related Sciences, 55(2), 59–63. https://tc.idm.oclc.org/login?url=https://search.ebscohost.com/login.aspx?direct=true&db=rzh&AN=132451529&site=ehost-live [PubMed] [Google Scholar]