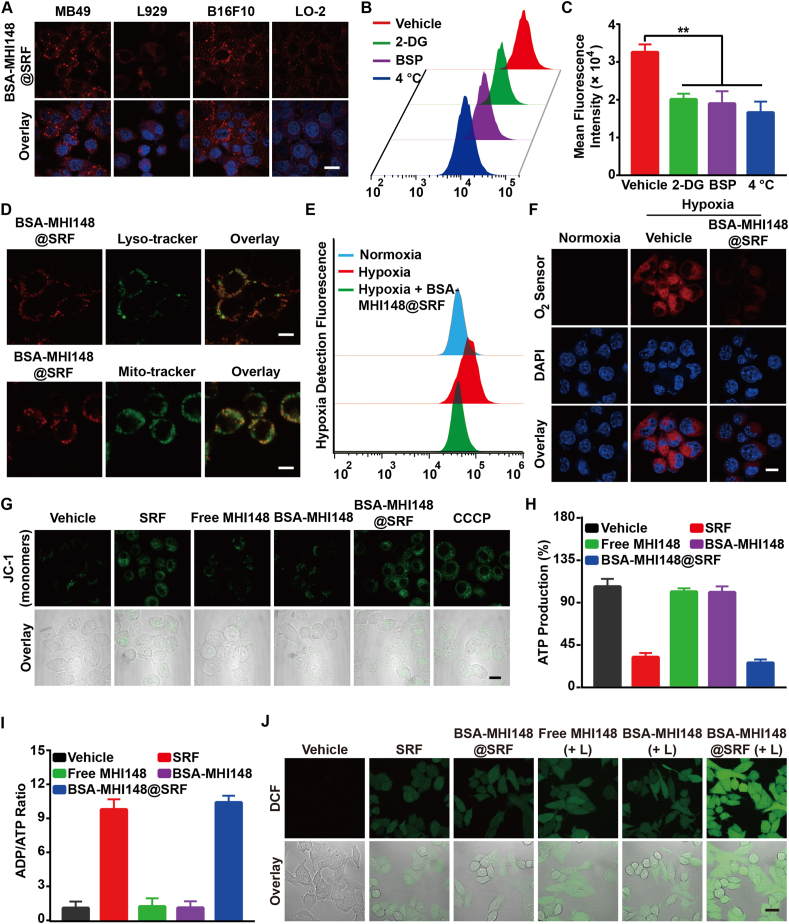
Figure 4.
BSA-MHI148@SRF nanoparticles inhibited mitochondrial respiration and enhanced ROS generation in vitro. (A) Laser confocal microscope images of BSA-MHI148@SRF nanoparticles uptake in tumor cells (MB49 and B16F10) and normal cells (L929 and LO-2), scale bar = 20 μm. (B, C) Mean fluorescence intensity of BSA-MHI148@SRF nanoparticles in MB49 cells pretreated with 2-deoxy-d-glucose (2-DG), sulfobromophthalein (BSP), and 4 °C (n = 3). (D) Colocalization of BSA-MHI148@SRF nanoparticles with Lysotracker Green-stained lysosomes and Mitotracker Green-stained mitochondria, scale bars = 10 μm. (E) The fluorescence of the O2 sensor (Hypoxia/Oxidative Stress Detection Kit) in MB49 tumor cells determined by flow cytometry. (F) Representative CLSM images of MB49 cells treated with BSA-MHI148@SRF nanoparticles in the hypoxia condition, the cells were then stained by an O2 sensor, scale bar = 20 μm. (G) Representative CLSM images of MB49 cells treated with BSA-MHI148@SRF nanoparticles and then stained by JC-1 kit, scale bar = 20 μm. (H, I) Detection of ATP content and ADP/ATP ratio in MB49 cells treated with PBS, SRF, free MHI148, BSA-MHI148, and BSA-MHI148@SRF nanoparticles, respectively (n = 3). (J) Representative CLSM images of the ROS generation detected by DCFH-DA in MB49 cells after different treatments, scale bar = 20 μm. Data are demonstrated as mean ± SD. Statistical analysis was performed via the two-tail Student’s t-test. ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001. (+ L) represented the tumor cells treated with 808 nm laser irradiation.