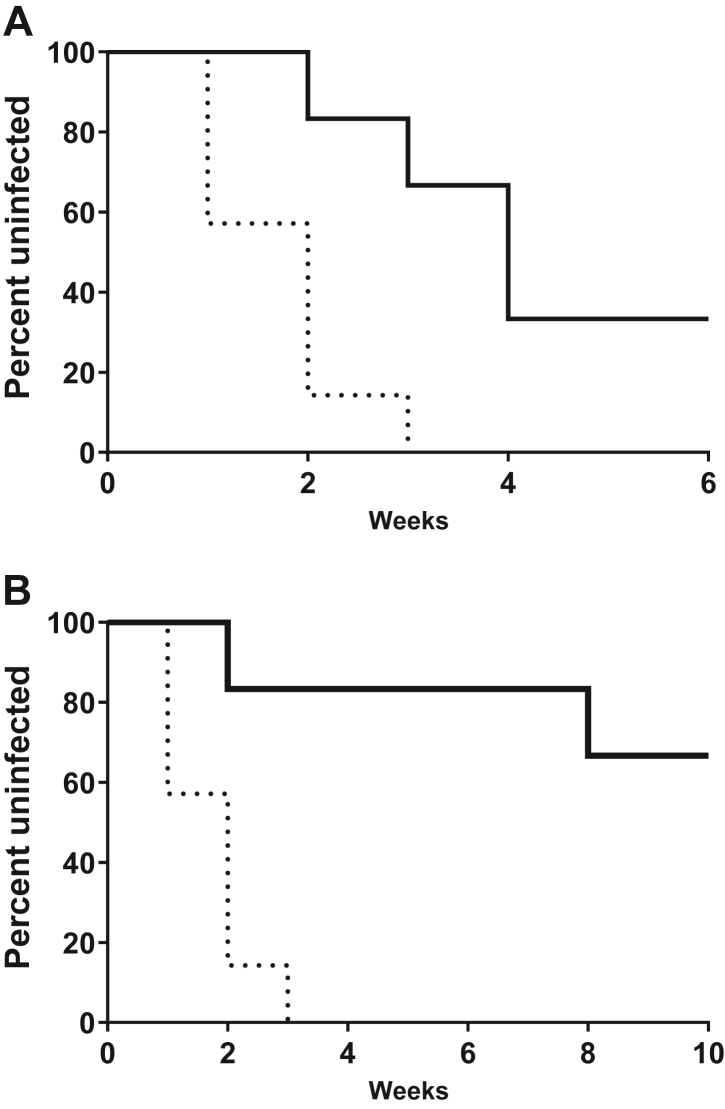
Fig. 4.
Rectal efficacy of TAF/EVG inserts administered 4h before SHIV exposure. Macaques received one or two weekly rectal inserts application and were exposed to SHIV162P3 at 4 h after dosing. The survival curve shows the cumulative percentage of uninfected macaques as a function of the number of weekly rectal SHIV162p3 exposures treated with one (a) and two (b) TAF/EVG inserts. Dashed black and solid black lines represent placebo controls (n = 7) and TAF/EVG insert (n = 6), respectively. Placebo controls were infected after a median of 2 exposures. TAF/EVG 1 insert animals were infected after a median of 4 exposures, and the calculated efficacy was 72.60%, with a 95% exact CI (24.47%, 92.66%); challenges were stopped after six weeks. TAF/EVG 2 inserts animals received 10 challenges. The calculated efficacy was 93.14%, with a 95% exact CI (73.26%, 99.21%). Animals in both arms were followed for an additional 5 weeks to monitor infection.