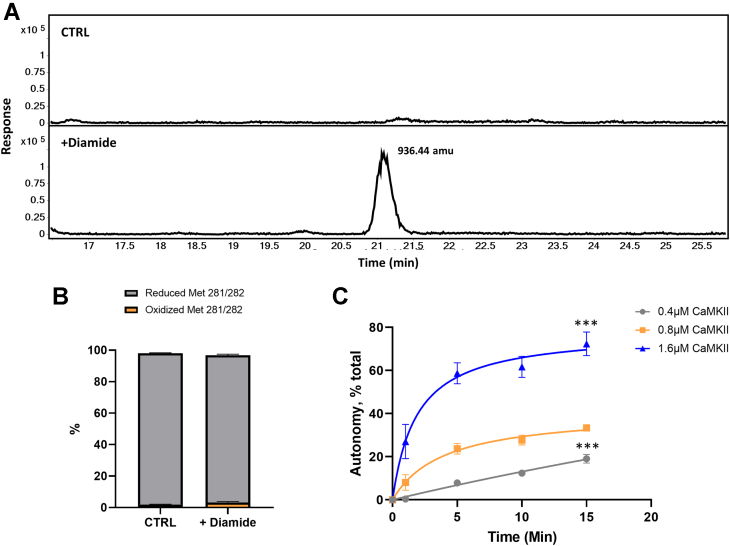
Figure 4.
Characterization of the disulfide formation induced by diamide.A, disulfide formation induced by diamide occurs between Cys273 and Cys290: CaMKII Cys273-Cys290 mutant was incubated or not with diamide and denatured and alkylated with iodoacetamide to label-free cysteine residues. After that, the enzyme was incubated with trypsin and then mapped by HPLC-MS. Only after diamide treatment (lower panel), two tryptic peptides were linked, residues 268 to 274 with Cys273 and residues 284 to 291 with Cys290. The calculated m/z is 936.44 amu. n = 3 replicates. B, methionine is not oxidized by diamide: methionine (gray) and methionine sulfoxide (orange) in the peptide containing Met281 and Met282 (residues 275–283) before and after incubation with diamide. n = 3 replicates. C, disulfide formation induced by diamide occurs between Cys273 and Cys290 from different CaMKII dodecamers: 0.4 μM (gray), 0.8 μM (orange), and 1.6 μM (blue). CaMKII Cys273-Cys290 mutant incubated with diamide during 1, 5, 10, and 15 min autonomous activity (after EGTA addition). n = 3 replicates. % of total activity (No EGTA addition). Data was fit to a hyperbola in Prism. ∗∗∗p < 0.01 compared with 0.8 μM CaMKII by unpaired t test. CaMKII, calcium/calmodulin-dependent protein kinase II.