Abstract
As an evolutionarily conserved signalling network, the Hippo pathway plays a crucial role in the regulation of numerous biological processes. Thus, substantial efforts have been made to understand the upstream signals that influence the activity of the Hippo pathway, as well as its physiological functions, such as cell proliferation and differentiation, organ growth, embryogenesis, and tissue regeneration/wound healing. However, dysregulation of the Hippo pathway can cause a variety of diseases, including cancer, eye diseases, cardiac diseases, pulmonary diseases, renal diseases, hepatic diseases, and immune dysfunction. Therefore, therapeutic strategies that target dysregulated Hippo components might be promising approaches for the treatment of a wide spectrum of diseases. Here, we review the key components and upstream signals of the Hippo pathway, as well as the critical physiological functions controlled by the Hippo pathway. Additionally, diseases associated with alterations in the Hippo pathway and potential therapies targeting Hippo components will be discussed.
Subject terms: Molecular biology, Molecular medicine
Introduction
The Hippo pathway was first discovered in Drosophila melanogaster and has been studied for the past 20 years. A timeline of essential discoveries and processes of the Hippo pathway is shown in Fig. 1. In mammals, the Hippo pathway is composed of several key components, including mammalian STE20-like kinase 1/2 (MST1/2), protein Salvador homologue 1 (SAV1), MOBKL1A/B (MOB1A/B), large tumour suppressor kinase 1/2 (LATS1/2), Yes-associated protein 1 (YAP), WW-domain-containing transcription regulator 1 (TAZ), and the transcriptional enhanced associated domain (TEAD) family1 (Fig. 2). YAP/TAZ are transcriptional coactivators that bind to TEAD1–4 to regulate the expression of a wide array of genes that mediate cell proliferation, apoptosis, and stem cell self-renewal.2 Moreover, a variety of upstream signals, such as cell polarity, mechanical cues, cell density, soluble factors and stress signals, modulate the Hippo pathway.3–5
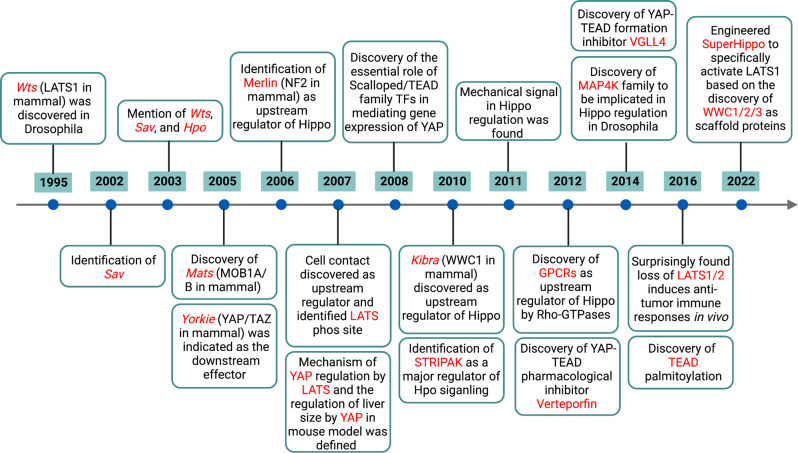
Fig. 1.
A timeline of essential discoveries and processes of the Hippo pathway. These discoveries were made initially in 1995 and then gradually to the present. The discoveries mainly focus on two aspects, including the components and processes of Hippo pathway and the function of Hippo pathway in physiological and pathological conditions
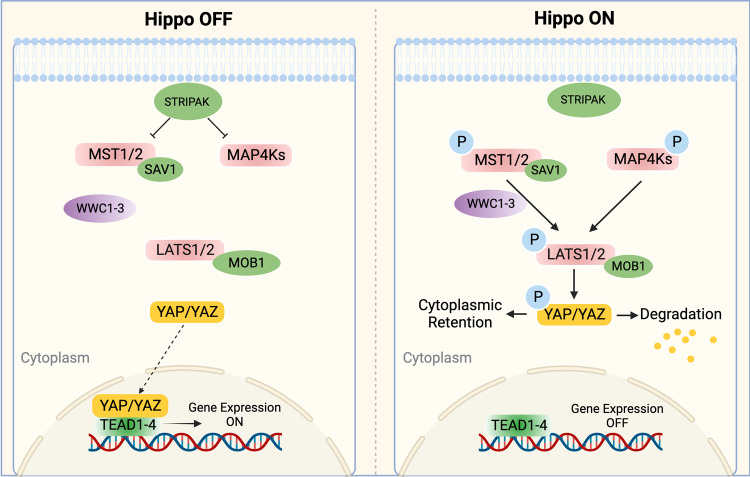
Fig. 2.
The core Hippo pathway in mammals. STRIPAK complex in the upstream regulates both MST1/2 and MAP4Ks. MAP4Ks or MST1/2 and its scaffold protein SAV1 could phosphorylate LATS1/2 and its scaffold MOB1 with the help of WWC1–3. The phosphorylated MOB1 can also directly promote the activation of LATS1/2 by inducing the conformational change of LATS1/2. The activated LATS1/2 phosphorylated and inactivated YAP/TAZ, preventing it from translocating into the nucleus and binding to transcription factors TEAD1–4
As a signalling pathway that modulates the proliferation, differentiation, and survival of cells, the Hippo pathway plays vital role in the development and homoeostasis of organs. Therefore, dysregulation of the Hippo pathway can cause a variety of diseases, including cancer,6,7 eye diseases,8,9 cardiac diseases,10,11 pulmonary diseases,12,13 renal diseases,14,15 hepatic diseases,16,17 and immune dysfunction.18,19 Thus, developing therapeutic approaches targeting Hippo components will expand the availability of precise therapies against cancers and other diseases. To date, a multitude of laboratory investigations has been performed to assess the therapeutic value of these strategies in vitro and in vivo. Furthermore, some of these strategies have already been evaluated in clinical trials.
In this review, key components, upstream signals of the Hippo pathway, and critical physiological functions controlled by this pathway will be discussed. In addition, studies focusing on the consequences of the dysregulated Hippo pathway and potential therapeutic strategies targeting Hippo components will be evaluated. Moreover, the outcomes of drugs that manipulate the activity of the Hippo pathway will be analysed.
Key components of the Hippo pathway
The core of the Hippo pathway is a kinase cascade, and MST1/2, SAV1, LATS1/2, YAP, and TAZ are considered the key components.20–22 In general, the striatin (STRN)-interacting phosphatase and kinase (STRIPAK) complex works upstream of kinase kinase kinase kinases (MAP4Ks) and MST1/2 and inhibits the Hippo pathway.23–26 However, when the Hippo pathway is activated, MAP4Ks, MST1/2 and its scaffold protein SAV1 phosphorylate LATS1/2 and its scaffold MOB1A/B.27 Then, activated LATS1/2 phosphorylates and inhibits YAP and TAZ, preventing them from translocating into the nucleus to interact with TEAD 1–4.28
MST1 and MST2 are serine/threonine kinases whose activity can be enhanced by being complexed with the scaffold protein SAV1 through their C-terminal SARAH (Sav/Rassf/Hpo) domains.29 MST1/2 also facilitates the binding between MOB1A/B and LATS1/2.30 Recently, Sixian Qi et al.31 showed that this process was mediated by WWC proteins (WWC1/2/3), which act as scaffold proteins. Besides, except for MST1/2, MAP4K family proteins have also been reported to participate in the activation of LATS1/2 without the direct involvement of SAV.32–35 Thus, the activation of MST1/2 or MAP4K proteins is an initiating signal for the Hippo pathway. The combined depletion of these three types of proteins has been shown to drastically block downstream signalling.36
MOB1 A/B plays dual roles in Hippo activation. First, MOB1A/B functions as a scaffold that contributes to the interaction between MST1/2 and LATS1/2. Second, phosphorylated MOB1 A/B can directly promote the activation of LATS1/2 by inducing a conformational change in LATS.37,38 LATS1 and LATS2 are serine/threonine kinases in the AGC kinase family. Upon activation, they directly interact with downstream YAP/TAZ. It was suggested that this interaction might be mediated by WW domains on YAP/TAZ and PxY motifs on LATS1/2.29,39,40 Compelling evidence suggests that activated LATS1 and LATS2 phosphorylate and inactivate YAP and TAZ,41 the main downstream effectors of the Hippo pathway.
When the Hippo pathway is activated, the activity of YAP/TAZ is inhibited through LATS1/2-mediated phosphorylation. When the Hippo pathway is inactivated, dephosphorylated YAP/TAZ translocates into the nucleus and binds to the transcription factors TEAD1-4 to induce gene expression.42 TEAD1-4 could function as transcriptional repressors by recruiting vestigial-like (VGLL) family proteins, such as VGLL343 and VGLL4.44,45 These factors competitively bind to TEAD and YAP/TAZ and cause transcriptional silencing. However, uncontrolled activation of YAP/TAZ and TEAD1-4 could lead to constitutive activation of this pathway, thereby leading to pathological consequences.46–48
Upstream signals of the Hippo pathway
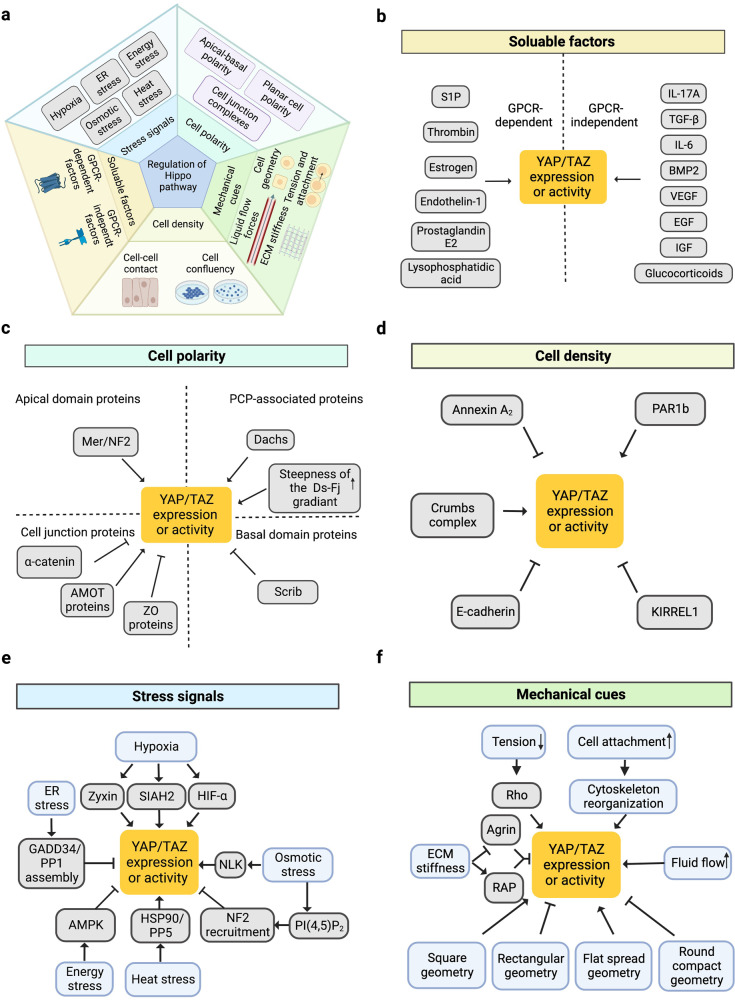
Because of the important biological roles of the Hippo pathway, considerable efforts have been made to examine the upstream signals that regulate the Hippo kinase cascade.5 To date, a large number of such signals have been identified.20,49–52 In this review, these signals are classified into five subgroups: cell polarity, mechanical cues, cell density, soluble factors, and stress signals53–57 (Fig. 3).
Fig. 3.
Regulation of the Hippo pathway by upstream signals. a Five subgroups of upstream signals including cell polarity, mechanical cues, cell density, soluble factors, and stress signals are responsible for the regulation of Hippo pathway. b–f The detailed upstream signals of Hippo pathway in every subgroup
Cell polarity
Cell polarity refers to distinct spatial characteristics with respect to the shape and structure of cells. It is well documented that the polarity of cells acts as a key regulator of the Hippo pathway.29 Generally, there are two types of cell polarity: apicobasal polarity (AP) and planar cell polarity (PCP). While AP divides the plasma membrane into the apical domain and basal domain,58 PCP refers to the polarization of epithelial cells along an axis perpendicular to the apical–basal axis (from proximal to distal).59
Notably, a wide spectrum of proteins on apical and basal domains or within the cell junction complexes have been reported to be upstream mediators of the Hippo pathway cascade.60 For example, in Drosophila, the tumour suppressor Merlin/neurofibromin-2 (Mer/NF2) localizes at the apical domain and functions as a linker for the actin cytoskeleton and plasma membrane.61 Mer/NF2 contributes to the recruitment of Hippo kinase and participates in the activation of the Hippo pathway.62 Additionally, posttranslational modifications of Mer/NF2, such as NEDD4L-mediated ubiquitination, have been shown to be required for the activation of Lats1.63 In mammals, it has been demonstrated that the cataract formation phenotype in NF2-deficient mice could be suppressed by the depletion of the Yap gene.64 In another study, the connection between NF2 and YAP was evaluated in the mouse myocardium.65 It was shown that the expression level of YAP was upregulated in the cardiomyocytes of cardiomyocyte-specific NF2-knockout mice. Moreover, NF2-deficient mice were resistant to H2O2-induced ischaemia/reperfusion (I/R) injury in the heart.65 In addition, Yap depletion could diminish the protection against I/R in cardiomyocyte-specific NF2-knockout mice, indicating that NF2 regulates the activity of YAP.65 Additionally, Scribble (Scrib) is a membrane protein localized on the basal domain. In Drosophila, the enhancement of cell migration induced by the inhibition of Scrib was shown to be mediated by Yki.66 Furthermore, it was shown that cancer-associated phenotypes may be induced by the interaction between Scrib and Yap, which inhibits the activity of YAP.67
In addition, apical and basal domains are physically separated by cell junction complexes. Many cell junction proteins have also been shown to play regulatory roles in the Hippo pathway by interacting with Hippo components.68,69. For example, the angiomotin (AMOT) family of proteins (AMOT, AMOTL1, and AMOTL2) are essential for tight junctions and cell polarity.70 It was reported that AMOT proteins function as scaffolds for LATS1/2, facilitating the phosphorylation of LATS1/2 by MST1/2. Moreover, AMOT contributes to the connection between LATS1/2 and YAP, which is required for the activation of YAP.71 In addition, α-Catenin is an essential component of the E-cadherin–catenin complex, and its function is of vital importance for the integrity of adherens junctions.72 It has been shown that α-Catenin can inhibit the nuclear localization of YAP. This inhibitory role is associated with the tumour-suppressive effects of α-Catenin.73 In addition, Zonula occludens (ZO) proteins (ZO-1, ZO-2, ZO-3) are scaffolding proteins that provide a structural basis for tight junctions.74 It has been shown that ZO-2 silencing leads to the activation of YAP and causes renal hypertrophy.75
Finally, PCP, another type of cell polarity, also functions as an upstream signal of the Hippo pathway. It has been demonstrated that PCP is regulated by the protocadherins Fat (Ft) and Dachsous (Ds),76,77 which are involved in the regulation of the Hippo pathway.78,79 Through the Golgi-resident kinase four jointed (FJ), Ft and Ds engage with each other heterophilically between cells. In addition, the Ft–Ds system functions as a ligand‒receptor pair for the Hippo pathway.5 Notably, it was shown that the regulatory effect of the Ft–Ds system on the Hippo pathway is regulated by the steepness of Ds–Fj gradients. While a shallow gradient activates the Hippo pathway, a steep gradient inhibits the activity of the Hippo pathway.80 Additionally, it was revealed that Dachs, an important downstream effector of Fat, plays key roles in the regulation of the Hippo pathway.81,82 It was reported that Dachs could influence the activity of the Hippo pathway by competing with Mats for binding to Warts.83
Mechanical cues
Mechanical cues are important signals by which cells sense their microenvironment. Through mechanotransduction systems, cells can translate mechanical cues into biochemical signals to control their behaviour. As early as 2011, Sirio Dupont et al.84 revealed the essential role of YAP/TAZ in the mechanotransduction system. This finding suggests a tight connection between the Hippo pathway and mechanical cues. Through the Hippo pathway, cells sense and respond to mechanical cues such as extracellular matrix (ECM) stiffness, cell geometry, liquid flow forces,85 tension and attachment. These mechanical cues have strong effects on the proliferation, survival, and differentiation of cells through the Hippo pathway.84
Changes in ECM stiffness represent an important type of mechanical cue. It was reported that the Ras-related GTPase RAP2 could be activated by low ECM stiffness, which leads to the activation of LATS1/2.86 Additionally, Agrin, an ECM proteoglycan that binds to lipoprotein-related receptor-4 (Lrp4) and muscle-specific kinase (MuSK), has been reported to relay matrix rigidity signals to the Hippo pathway by interrupting the functioning of Merlin and LATS1/2.87 Moreover, it was revealed that the aberrant expression of tenascin C leads to the repression of ECM adhesion forces and activation of the Hippo pathway, thereby facilitating new bone formation.88
Additionally, changes in cell geometry could affect the activity of the Hippo pathway cascade. It has been observed that YAP/TAZ tends to localize in the nucleus in murine myoblasts with a rectangular shape.89 When myoblasts are in an elongated rectangular shape, the ratio of cytoplasmic to nuclear YAP/YAZ is increased.89 This finding suggests that the geometry of cells can affect the distribution of YAP/TAZ by regulating the activity of the Hippo pathway. In addition, in NIH-3T3 mouse embryonic fibroblasts, similar geometry-mediated regulation of the Hippo pathway was identified. It was shown that YAP accumulates in the nucleus in flat spread cells, while in round compact cells, YAP localizes in the cytoplasm.90
Liquid flow force represents another upstream mechanical cue that regulates the Hippo pathway. It is noteworthy that a substantial proportion of human body fluids are flowing liquids such as blood and lymph.91 As a consequence, many cells are exposed to different levels of liquid shear stress. Compelling evidence suggests that cells respond to liquid shear forces through the Hippo pathway.92,93 The first study that uncovered the relationship between liquid shear forces and the regulation of the Hippo pathway revealed that increased fluid flow promoted the expression of YAP and was associated with osteogenesis and a decrease in adipogenesis in mesenchymal stem cells (MSCs). In chondrocytes, increased fluid flow leads to increased expression of YAP and results in dedifferentiation.94
Cytoskeleton tension and cell‒cell attachments, which are mechanical forces, are also implicated in the upstream regulation of the Hippo pathway. Several regulator proteins, such as Rho, jub and Ajuba LIMD1, take part in the transmission from tension and attachment of cells to the Hippo pathway. It was reported that Rho participates in the regulation of cell attachment-induced YAP dephosphorylation.95 Moreover, it was shown that the activity of Rho plays a pivotal role in the cell attachment-dependent regulation of the Hippo pathway.96 The protein Jub, which can negatively regulate Warts within the Hippo pathway, was shown to regulate Yki activity in response to cytoskeletal tension.97 In addition, the Ajuba family protein LIMD1 and the contractile protein spectrin participate in cytoskeleton tension-mediated Hippo pathway changes.96,98
In summary, different mechanical cues are involved in the upstream regulation of the Hippo pathway. Although it has been found that integrin,99 peizo100 and plexin101 can act as mechanical sensor proteins that transmit signals to the Hippo pathway, the comprehensive molecular mechanism by which cells sense mechanical signals and change cellular behaviours through the Hippo pathway is still unclear. The proteins in cell‒cell contact sites and the cytoskeleton might play critical roles in the signal transmission between mechanical cues and the Hippo pathway cascade.102 Notably, although the mechanisms by which mechanical cues affect the Hippo pathway cascade have yet to be fully understood at the molecular level, one of the core components of the Hippo pathway, YAP/TAZ, is a vital mediator of mechanical cues.103
Cell density
It is frequently observed that the proliferation rate of cells is negatively correlated with cell density in the monolayer culture. This phenomenon was shown to be associated with cell–cell contact.104 It was shown that high confluence of mammalian cells leads to the activation of LATS and the phosphorylation and inactivation of YAP. Furthermore, the overexpression of YAP could reverse the growth inhibition induced by cell density, suggesting a critical role of YAP in cell contact inhibition.105
The mechanisms by which the Hippo pathway is regulated by cell density have yet to be completely elucidated. Thus far, several complexes or proteins have been reported to function as sensors that transmit cell density signals to the Hippo pathway.5,106 It has been demonstrated that cell density can be sensed by the Crumbs complex, which includes AMOT. The Crumbs complex interacts with YAP/TAZ and facilitates its phosphorylation. This phosphorylation results in the suppression of the TGF-β-SMAD signalling pathway and leads to epithelial-to-mesenchymal transition.68 A recent study revealed that Kirre-like Nephrin Family Adhesion Molecule 1 (KIRREL1), a cell adhesion molecule, acts as a feedback regulator of the Hippo pathway in mammalian cells. It was shown that KIRREL1 could sense cell‒cell interactions and mediate the recruitment of SAV1 to cell‒cell contact sites. KIRREL1 knockout led to the activation of YAP.107,108 Recently, integrated screens revealed KIRREL as a cell surface tumour suppressor involved in the Hippo pathway that could bind directly to SAV1 to activate this pathway.109 In addition, it was shown that the palmitoylation of TEAD was also regulated by cell density.110 Reportedly, other cell density transmitters for the Hippo pathway include E-cadherin,111 annexin A2,112 and the polarity-regulating kinase PAR1b.113
Soluble factors
Soluble factors regulate the majority of biological and physiological processes. To date, numerous soluble factors have been shown to influence the activity of the Hippo pathway.4,114 Notably, G protein-coupled receptors (GPCRs) make up the largest family of membrane receptors for soluble factors in mammals.115 The Hippo pathway has been shown to be regulated by GPCR signalling.116–119 For example, it was shown that GPCR ligands such as thrombin or lysophosphatidic acid (LPA) could activate YAP in fibroblasts and sensitize them to TGF-β1.120 Additionally, sphingosine-1-phosphate (SIP) is a ligand of GPCR. SIP can induce the nuclear localization of YAP and promote the expression of YAP target genes in mouse embryonic cells and liver cells.119 In addition to thrombin, LPA, and S1P, a large number of soluble factors have been shown to regulate the Hippo pathway by interacting with GPCRs, such as Oestrogen,121 Endothelin-1,122 Angiotensin II,123 and Prostaglandin E2.124 Additionally, an investigation by Rui Gong et al.125 showed that protein kinase C (PKC) is one of the major effectors downstream of GPCRs that modulate YAP activity.
In addition, some soluble factors have been shown to affect the Hippo pathway independent of GPCRs. For example, it was reported that IL-17A could induce the recruitment of MST1 to TRAF3 interacting protein 2 (TRAF3IP2) in HaCaT and NHEK cells. Then, the MST1–LATS1 interaction is inhibited, leading to the dephosphorylation of YAP. This mechanism has been shown to facilitate cell proliferation in psoriasis.126 Furthermore, glucocorticoid receptor signalling has been shown to participate in the regulation of the Hippo pathway. Glucocorticoids were shown to elevate the expression of fibronectin, thus leading to cytoskeleton-dependent YAP activation in human breast cancer.127 Additionally, transforming growth factor-beta (TGF-β) was shown upregulate TAZ levels in mesenchymal and epithelial cells. A mechanistic study revealed that inhibiting p38 MAPK signalling suppressed TAZ upregulation in response to TGF-β.128 In addition, supressing myocardin-related transcription factor (MRTF) represses TAZ upregulation induced by TGF-β. These results suggest that TGF-β can regulate the Hippo pathway through p38- and MRTF-mediated signalling.128 Other soluble factors that influence the activity of the Hippo pathway include bone morphogenic proteins,129 IL-6,130 insulin/insulin-like growth factors,131 epidermal growth factors,132 and vascular endothelial growth factors.133
Stress signals
Cellular stress, such as hypoxia, endoplasmic reticulum (ER) stress, energy stress, osmotic stress or heat stress, can act as upstream signals of the Hippo pathway, subsequently regulating the behaviours, survival, and metabolism of cells. The mechanisms by which cells sense and transmit these signals to Hippo components have been extensively examined.134–136
Hypoxia is a condition in which the cell has a limited oxygen supply. In epithelial ovarian cancer cells, it was observed that 1% O2 or hypoxia mimics downregulated YAP phosphorylation (S127) but upregulated TAZ phosphorylation (S69), suggesting that hypoxic conditions could differentially mediate the activities of YAP and TAZ.137 Additionally, it has been reported that the regulation of the Hippo pathway by hypoxia is mediated by Zyxin,138 SIAH2 ubiquitin E3 ligase,139 and hypoxia-inducible factor 1 subunit alpha (HIF-1α).140 ER stress is induced by the accumulation of misfolded proteins in the ER when cells are exposed to an unstable or adverse environment.141 In human hepatocellular carcinoma cells, ER stress has been shown to inhibit the activity of YAP and enhance apoptosis by promoting the assembly of the GADD34/PP1 complex.142 Energy stress is characterized as a disruption of the homoeostasis of cellular energy. AMP-activated protein kinase (AMPK) functions as a sensor of energy stress. It has been demonstrated that energy stress could induce AMPK-dependent Lats activation and lead to the phosphorylation of YAP.143,144 This finding has been used to explain the observation that metformin, an antidiabetic drug that interacts with AMPK, exerts anticancer effects.134 Additionally, osmotic stress caused by sorbitol treatment can induce a dynamic balance between YAP activation and inhibition. In 2017, Hong et al.145 discovered that osmotic stress induced the phosphorylation of YAP at Ser128 by Nemo-like kinase (NLK), which then interfered with its binding with 14-3-3, resulting in YAP nuclear accumulation and activation. Moreover, osmotic stress inhibits YAP through phosphorylation at Ser127, and the underlying molecular mechanism was further investigated by the same team in 2020.146 The researchers found that osmotic stress could change the cell membrane distribution of phosphatidylinositol-4,5-bisphosphate [PI(4,5)P2], leading to the plasma membrane recruitment of neurofibromin 2 (NF2), also known as merlin, to induce downstream Hippo pathway activation.146 Moreover, heat stress is an important upstream signal of the Hippo pathway. Min Luo et al.147 revealed that heat stress inhibited LATS kinase by interacting with HSP90 and PPP5, thereby activating YAP/TAZ to induce the heat shock transcriptome.
Critical physiological functions of the Hippo pathway
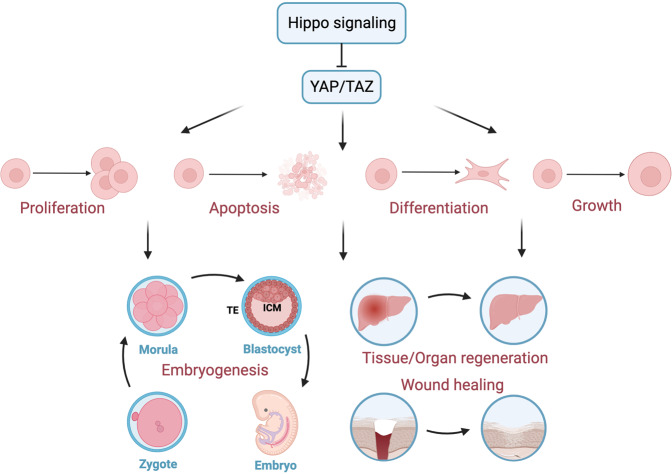
While the Hippo pathway first drew attention for its critical role in the control of organ size in Drosophila, over a decade of intense research has confirmed its widespread physiological roles in human health, ranging from decisions regarding cell fate determination during embryonic development to tissue/organ regeneration and wound healing (Fig. 4).
Fig. 4.
The essential physiological function of Hippo pathway. The Hippo pathway effectors YAP/TAZ can take part in the modulation of multiple cell events, including proliferation, apoptosis, differentiation and growth, thereby participating in the physiological processes of embryogenesis and development, as well as tissue/organ regeneration and wound healing
Cell growth, proliferation and differentiation
YAP/TAZ are key downstream effectors of the Hippo pathway that can translocate to the nucleus to induce the TEAD-mediated expression of genes related to cell growth and proliferation.21,148
The Hippo pathway has been shown to restrict the proliferation of cardiomyocytes,10,149 the molecular mechanism in which involves the Wnt150 or Pi3kcb-mediated PI3K-AKT151 signalling pathways. The dystrophin–glycoprotein complex (DGC) was further shown to inhibit cardiomyocyte proliferation by directly binding with Yap.152 Marta Diez et al.153 showed that 96 screened miRNAs could stimulate human iPSC-derived cardiomyocyte replication by inhibiting the Hippo pathway. In addition, in human and mouse skin, YAP-TEAD activation promotes the proliferation of keratinocytes to maintain skin homoeostasis154,155 or mediate IL-17A-driven psoriasis.126 Moreover, the Hippo pathway regulates contact-dependent cell growth and proliferation in cancer cells,156,157 nervous Schwann cells,158 epidermal stem cells159 and hepatic160 or lung epithelial cells.161,162
In addition to the role of the Hippo pathway in cell growth/proliferation control, this pathway has also been shown to affect specific cell differentiation in a variety of tissues and organs, including the pancreas, lung, muscle and mammary glands. For example, pancreatic-specific Mst1/2-knockout mice were shown to exhibit acinar cell dedifferentiation to ductal cells, which was mediated by the hyperactivation of YAP.163 In the developing human lung, Mst1/2 deletion and YAP activation were first shown to affect epithelial progenitor cell differentiation.164,165 In addition to airway epithelial progenitors, YAP is also required for the differentiation of proximal airway166 or airway basal stem cells167 in adult lungs. The role of YAP-LATS in the differentiation of type I168 or type II169 alveolar epithelial cells was further confirmed in bronchopulmonary development. However, YAP overexpression promotes myoblast differentiation170 and conversely represses the differentiation of satellite cells of skeletal muscle.171 Further studies indicated that the absence of YAP/TAZ causes the loss of the differentiated contractile phenotype in vascular smooth muscle cells (VSMCs) and osteogenic differentiation.172 Chen Q et al.173 identified that Sav1 deletion or Yap overexpression could prevent the differentiation of mammary cells. In contrast, McNeill et al.174 discovered that YAP/TAZ activation by Lats1/2 deletion could promote the differentiation of nephron progenitor cells into interstitial myofibroblastic cells in the kidney. In addition to the aforementioned cells, the Hippo pathway has also been reported to regulate specific cell differentiation in other tissues or organs, such as skin keratinocytes,154 intestinal epithelial cells,175 hepatocytes and biliary cells.176,177
Overall, these findings suggest that Hippo pathway components, especially YAP/TAZ, are involved in cell growth/proliferation and differentiation in different tissues or organs under diverse contexts.
Embryogenesis and development
Embryogenesis in mammals involves several essential stages, including preimplantation, gastrulation, neurulation and organogenesis. The formation of the blastocyst is one of the important events in the preimplantation stage, which consists of the outer epithelial trophectoderm (TE) and the inner cell mass (ICM). The specification of TE and ICM is a key event in early embryogenesis,178 and many studies have revealed the contribution of Hippo components to this process. For example, nuclear YAP was shown to be restricted in outside cells and drives TEAD4-mediated cdx2 expression to take part in TE specification.179 Similarly, GATA binding protein 3 (GATA3) was shown to be regulated by TEAD4 to promote the differentiation into TE.180 Unlike the outside cells of the morula, WWTR1/YAP1 was repressed by LATS1/2 to permit the expression of SOX2 in the inside cells. The deletion of LATS1/2 leads to the accumulation of nuclear YAP in inner cells and causes abnormalities in the ICM and changes the cell fate towards TE-like.181,182 Similarly, inhibiting the upstream actor NF2 causes the mislocalization of YAP and alters the production of CDX2 inside cells.183 In summary, in morula, the Hippo pathway is highly activated in inside cells, whereas Hippo is in a low activation state in outside cells to maintain the normal specification of TE and ICM in the preimplantation stage.
Following the preimplantation stage, embryo development enters the gastrulation and neurulation stage on Day 26 in humans and embryonic Days 6 (E6) to 9 (E9) in mice.178 YAP−/− embryos showed defects in the yolk sac vasculature on E8.5, which indicates that the Hippo pathway may contribute to angiogenesis in the gastrulation and neurulation stages.184 The relationship between angiogenesis and the Hippo pathway has already been widely discussed.133,185 However, the detailed underlying mechanism by which Hippo contributes to angiogenesis in this stage is unclear and should be further studied.
After the gastrulation and neurulation stages, at approximately 3–8 weeks in humans, organogenesis occurs.178 It has already been shown that the development of organs such as the heart, lung, and kidney is related to the Hippo pathway. In terms of cardiac development, von Gise et al.186 reported that YAP could contribute to cardiac development by inducing cardiomyocyte proliferation. Deletion of Yap1 in foetal cardiomyocytes leads to lethal cardiac hypoplasia. Conversely, inactivation of the Hippo pathway, such as through Yap1 activation or Salv, Lats2 and Mst1/2 knockout, causes cardiomegaly.150,186 For lung development, YAP is essential for the proper morphogenesis of the airway. Severe branching morphogenesis disruptions occur when the expression of YAP is blocked.187 Moreover, the deletion of both Mst1 and Mst2 causes severe lung abnormalities, resulting in death at birth.164 Furthermore, the Hippo pathway plays an important role in kidney development. It has already been reported that YAP is essential for nephrogenesis, while NF2 and LATS are needed for the morphogenesis of ureter branching.188,189
Tissue/organ regeneration and wound healing
The Hippo pathway plays pivotal roles in tissue/organ regeneration and wound healing.190 While tissue/organ regeneration is an important biological process that makes tissues/organs resilient to damage and disturbances, wound healing refers to the process by which the skin repairs itself after injury.191 Recent evidence suggests that YAP/TAZ, the key component of the Hippo pathway, is activated after damage to the skin or a variety of organs, such as the intestine, liver, heart, and lung.192
The role of the Hippo pathway in intestinal homoeostasis and regeneration is controversial and multifaceted.192 Generally, YAP/TAZ is crucial and indispensable for intestinal tissue regeneration after injury in both Drosophila and mice.193 Many studies have reported that the level of YAP protein in the intestinal epithelium is highly increased during intestinal regeneration and that its inactivation severely compromises this regenerative programme.194,195 In addition, the self-renewal and regeneration of the intestine are dependent on intestinal stem cells (ISCs), which are positive for leucine-rich repeat-containing GPCR5 (Lgr5)196 and mainly express YAP.194 YAP promotes intestinal regeneration by suppressing Wnt signalling in Lgr5+ ISCs.197 Conversely, an inhibitory effect of YAP on intestinal regeneration was discovered by Barry et al.194. The discrepancy in Wnt inhibition by YAP/TAZ may contribute to the inconsistent results. Furthermore, the inactivation of YAP/TAZ in mouse intestines resulted in no visible abnormalities, suggesting that YAP is not indispensable for normal intestinal development and homoeostasis.195,198
In the liver, Yap1 activation in hepatocytes contributes to liver regeneration.199 Additionally, the nuclear accumulation of Yap1 was increased in proliferating hepatocytes after partial hepatectomy, which facilitated the epithelial–mesenchymal transition (EMT) for liver regeneration.200 Additionally, blocking MST1/2 effectively enhanced liver repair and regeneration.201 It is commonly believed that the regenerative capacity of mammalian hearts is lost after the neonatal stage.202 However, recent evidence suggests that YAP activation can induce cardiac regeneration in adult mice.10,190,203,204 Several investigations have been conducted to examine the mechanisms by which YAP/TAZ mediate cardiac regeneration. For example, Yap activates the insulin-like growth factor (IGF) signalling pathway to augment the proliferation of cardiomyocytes.205 In addition, Yap activation was shown to be associated with EMT during cardiac regeneration.206 In the context of lung regeneration, it was reported that in alveolar stem cells, Yap is activated after pneumonectomy, which plays a critical role in alveolar regeneration.207 Moreover, it was observed that lung regeneration is substantially delayed in mice that lack Yap/Taz in alveolar epithelial type II cells.208 In addition to these organs, activation of YAP/TAZ has been shown to contribute to regeneration in many other tissues, such as the nervous system209–211 and bone.212
The roles of the Hippo pathway in wound healing have been extensively examined.213–215 Notably, the Hippo pathway affects wound healing through various mechanisms. For example, the activation and nuclear localization of YAP/TAZ promote proliferation in epithelial cells during wound healing.213,216 Additionally, TEAD inhibition increases Kruppel-like factor 4 (KLF4) levels and disrupts skin homoeostasis, thus impairing wound healing.154 In addition, wound-healing-related epithelial–mesenchymal transition (EMT) was shown to be regulated by YAP/TAZ in mice.217 Furthermore, the inhibition of YAP was shown to promote the expression of IL-33 and then lead to autophagy inhibition, which contributes to wound healing.218
Collectively, embryonic development and adequate and efficient tissue regeneration require highly controlled and harmonious cell proliferation, as well as cell differentiation. Considerable efforts should be devoted to understanding the complex molecular mechanisms by which YAP/TAZ mediate these critical physiological functions.
Dysregulation of the Hippo pathway and human diseases
As a signalling pathway that governs the proliferation, differentiation, and survival of cells, the Hippo pathway plays a vital role in the development and homoeostasis of organs. Therefore, dysregulation of the Hippo pathway causes a variety of diseases, including cancer, eye diseases, cardiac diseases, pulmonary diseases, renal diseases, hepatic diseases, and immune dysfunction (Fig. 5). In this section, the consequences of aberrant Hippo pathway function in human diseases will be discussed.
Fig. 5.
The summary of diseases caused by the dysregulation of the Hippo pathway. Hippo pathway dysregulation has been found to be present in a variety of organs or systems diseases and involved in the regulation of occurrence or progression of these diseases. The specific diseases are shown in boxes
The Hippo pathway in cancer
The hypothesis that the Hippo pathway has a close connection with cancer was initiated by the discovery that the egregious overgrowth of Drosophila melanogaster tissues could be caused by Hippo gene mutations.219–221 Overwhelming evidence suggested that the Hippo pathway was one of the most frequently dysregulated pathways in human cancer; YAP/TAZ were commonly identified as oncoproteins, while MST1/2 and LATS1/2 were identified as tumour suppressors.222–225 Cancers such as uveal melanoma,226 mesothelioma,227 ependymoma,228 and NF2-related schwannomas229 have all been shown to be related to Hippo pathway dysregulation. Generally, the Hippo pathway can affect human cancer in three ways: tumour initiation and progression, tumour metastasis, and tumour drug resistance. The detailed underlying mechanisms will be discussed below.
The Hippo pathway in tumour initiation and progression
Tumour initiation and progression is a multistep process that is characterized by the transformation of normal cells to malignant tumour cells and is triggered by multiple factors. One of these factors is the dysregulation of signalling pathways related to cellular survival.230 Because the Hippo pathway is an essential survival-associated signalling pathway, inactivation of this pathway could increase cell proliferation and decrease apoptosis, contributing to tumour initiation and progression.3,219 Tumour initiation and progression require metabolic reprogramming.231 Some researchers have reported that the Hippo pathway participates in cancer-related metabolic reprogramming, such as glycolysis, which requires tumour cells to obtain the necessary energy and building blocks and can be promoted by active YAP.144,232
Cancer stem cells (CSCs), which are a subpopulation of cancer cells, play an important role in tumour initiation and progression.233 In a recent study, the Hippo pathway component TAZ potentiated CSCs. Depletion of TAZ significantly decreased the tumour-seeding ability.234 Thus, the Hippo pathway contributes to tumour initiation and progression by regulating CSCs.
However, a few recent studies have presented some opposing ideas. In certain types of tumours, the Hippo pathway may change from a tumour suppressor to a tumour promoter. Cheung et al.235 found that YAP plays a suppressive role in colorectal tumour growth. YAP overexpression hindered both primary and metastatic colorectal cancer. Similar tumour suppressive activity of YAP/TAZ was observed in ER+ breast cancer,236 haematological cancers237 and several solid cancers of neural/neuroendocrine origin.238 In addition, deletion of the tumour suppressor’ LATS1/2 in cancer cells inhibits tumour growth in B16, SCC7 and 4T1 immunocompetent syngeneic mouse models in vivo due to enhanced immunogenicity of the cancer cells.41 These findings suggest that the Hippo pathway may play a tissue type-specific role in tumorigenesis. Further evaluation of this relationship in the occurrence and development of different types of tumours is essential for the development of precise Hippo-related treatments.
The Hippo pathway in tumour metastasis
Tumour metastasis is known as a multistep process that is associated with a higher level of malignancy. Accumulating evidence suggests that Hippo pathway components, including LAT1/2, MST1/2, YAP and TAZ, may play important roles in influencing tumour metastasis.235,239–244 Furthermore, the Hippo pathway was shown to influence various types of metastases. Lung and lymph nodes are two common sites of breast cancer metastasis, while the brain is a common site of lung cancer metastasis.245,246 in YAP-deficient PyMT mice, which is a breast cancer model, the incidence of lung metastasis was reduced.173 In a breast tumour with high lymph node metastasis, the expression of LATS1/2 decreased cancer metastasis.241 In addition, YAP inhibition could markedly decrease H2030-BrM3 cell brain metastasis in vivo.247 In most cases, the Hippo pathway may act as an inhibitor of tumour metastasis; thus, it represents a potential target for antitumor metastasis therapies.
The underlying mechanism by which the Hippo pathway affects tumour metastasis can mainly be divided into two parts. First, the Hippo pathway can regulate the migration and invasion of cells. Epithelial-to-mesenchymal transition (EMT) is a significant characteristic of cancer cells with enhanced migration.248 Activated YAP/TAZ can increase the expression of EMT-related transcription regulators.20,249 In contrast, Hippo pathway inactivation can play suppressive roles against the migration and invasion of cells. For example, YAP knockout promotes breast cancer lung metastasis.194,250,251 Second, the Hippo pathway could contribute to tumour metastasis by suppressing anoikis, a form of apoptosis induced by the loss of attachment between cells and the ECM. It was revealed that LIM domain only 3 (LMO3) could inhibit anoikis to promote hepatocellular carcinoma metastasis by suppressing the Hippo pathway.252
The Hippo pathway in the development of tumour drug resistance
Multiple therapies have been developed to treat cancers. In particular, chemotherapy, immunotherapy, and targeted therapy are the three main anticancer therapies. However, the efficacy of these treatments is severely impaired due to tumour drug resistance.253–255 Compelling evidence has shown that the Hippo pathway contributes to the development of chemotherapy resistance. The main four Hippo components, YAP, TAZ, MST1 and LATS1/2, were all shown to take part in the development of chemoresistance.256 For example, YAP and TAZ overexpression or YAP nuclear translocation could decrease the efficacy of cisplatin,257, doxorubicin,258 5-fluoracil259,260 and Taxol,261 while MST1 and LATS1/2 downregulation resulted in resistance to cisplatin262 and 5-fluoracil.263
The underlying mechanism of chemotherapy resistance may be connected to the stemness of cancer cells or drug metabolism and efflux. First, the Hippo pathway could affect chemotherapy resistance by regulating CSCs. CSCs, which are tumour-initiating cells, are a subgroup of cancer cells that could contribute to chemoresistance. The Hippo pathway is an important pathway that regulates CSCs234,264–266. In ovarian cancer, the overexpression of miR-30b and the downregulation of MYPT1 were shown to cause the expansion of CSCs by inactivating the Hippo pathway, ultimately resulting in platinum resistance264. In addition, the Hippo pathway was shown to be related to drug metabolism and efflux, which is an important factor in determining the efficacy of chemotherapy drugs. The increased efflux and metabolic conversion of a drug will decrease its efficacy considerably by reducing intracellular drug concentrations267. It was reported that YAP activation could sensitize pancreatic cancer cells to gemcitabine by downregulating drug efflux transporters and decreasing the conversion of gemcitabine from a less active form to an active form, resulting in increased concentrations of gemcitabine in tumour cells268.
In addition to chemotherapy, immunotherapy can be affected by the Hippo pathway. Over the last decade, immunotherapy has been widely examined. Immune checkpoint inhibitor therapy and chimeric antigen receptor (CAR) T-cell immunotherapy are the most striking examples269–271. However, the efficacy of these therapies still faces challenges associated with resistance. Myeloid-derived suppressor cells and tumour-associated macrophages are immunosuppressive cells in the tumour microenvironment that can contribute to immunotherapy resistance272–274. It was reported that the Hippo pathway could influence these two cell types to reduce the efficacy of immune therapy. YAP can directly induce cytokines such as CXCL5 and CCL2, which attract myeloid-derived suppressor cells and tumour-associated M2 macrophages, respectively, to confer resistance to immunotherapy275,276.
Finally, the most important clinical implication is the involvement of the Hippo pathway in targeted therapy resistance. There are currently several targeted cancer therapies, such as BRAF inhibitors, MEK inhibitors and epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors. BRAF inhibitors can be used to treat BRAF-mutant melanoma. However, efficacy could be limited because of drug resistance.277,278 While exploring the mechanism of BRAF inhibitor resistance in melanoma, researchers found that the upregulation of MOB3B and activation of the Hippo pathway contribute to vemurafenib resistance.279 In addition, NF2 is involved in vemurafenib resistance.280 Regarding MEK inhibitor resistance, the Hippo pathway may play an essential role. In vitro, the A549 and HCC44 cells that express YAP1 5SA were shown to have selumetinib resistance.281 The combination of YAP suppression and MEK inhibition can induce apoptosis in NSCLC, melanoma, colon cancer and thyroid cancer harbouring BRAF V600E, while the MEK inhibitor alone can not induce this effect.282 Clinically, the increased YAP levels may decrease the efficacy of MEK inhibitors.282 Ultimately, in terms of EGFR tyrosine kinase inhibitor resistance, TAZ expression is one of the intrinsic mechanisms. The overexpression of TAZ in PC9 cells reduced their sensitivity to gefitinib. TAZ knockdown sensitized gefitinib-resistant PC9/GR cells to gefitinib.283 Moreover, other targeted therapies, such as mTOR and CDK4/6 inhibition therapy, were shown to be related to the Hippo pathway.284,285
The Hippo pathway in eye diseases
The connection between the Hippo pathway and the development of the eye has been studied for many years. In many ocular tissues such as the cornea, lens and retina, YAP was shown to be ubiquitously distributed.286 Additionally, it was reported that the Hippo pathway may play an indispensable role in regulating retinogenesis, retinal neurogenesis, retinal angiogenesis, and corneal wound healing, suggesting that the Hippo pathway is involved in the regulation of ocular development.287–290 Therefore, dysregulation of the Hippo pathway substantially disrupts eye homoeostasis and results in different types of eye diseases.
In general, changes in the Hippo pathway in eye diseases are relatively complex. Hippo pathway dysregulation seems to be common in retinal-related diseases. In the retinas of mice with diabetic retinopathy, LATS and TAZ were increased, and p-MST and p-YAP were significantly decreased.291 In addition, in patients with Sveinsson’s chorioretinal atrophy, the Tyr421His mutation in TEAD1 has been found, and this mutant Tyr421His TEAD1 has a compromised interaction with YAP.148,292 Moreover, MST2 but not MST1 was identified as a factor that causes retinal detachment-induced photoreceptor cell death. MST2 deficiency could prevent photoceptor cells from death after retinal detachment.293
In addition to retinal-related diseases, the heterozygous inactivation of YAP1, which decreases the expression of YAP1 protein in lens epithelia, results in cataracts with lens epithelial cell defects.294 Moreover, NF2 deficiency in the lens epithelium in mice could lead to a cataract phenotype.64
The Hippo pathway in cardiac diseases
As early as 2011, a series of studies uncovered the role of the Hippo pathway in regulating heart size.150,205 Moreover, Wei Yu et al. identified the vital role of VGLL4 in heart valve development. The deletion of VGLL4 in mice could lead to serious valve malformation.295 These studies resulted in further studies on the connection between cardiac regulation and the Hippo pathway. Among them, the effect of the Hippo pathway on cardiac diseases is a hot topic. Cardiac diseases that are associated with a dysregulated Hippo pathway could be divided into at least four types: cardiomyopathy; heart failure; coronary heart diseases; and myocardial infarction.296
Cardiomyopathy is a group of cardiac diseases that are characterized by myocardial dysfunction. Hypertrophic cardiopathy (HCM), arrhythmogenic cardiomyopathy (ARCV) and dilated cardiomyopathy (DCM) are three common cardiomyopathies.297 All are associated with Hippo pathway dysregulation. Samples from patients with HCM were investigated, and YAP was shown to be increased at both the protein and mRNA levels. Moreover, the inhibitory phosphorylation at Ser127 of YAP was decreased, indicating the activation of YAP.298 However, the condition seems to be different in ARCV299 and DCM,300 in which the Hippo pathway was activated, leading to YAP inactivation.
Heart failure is a functional and structural heart disorder with complex clinical syndromes that can be affected by the Hippo pathway. p-YAP and p-LATS were increased in ischaemic or nonischaemic heart failure samples.301 In addition, TEAD1 was reported to take part in the dedifferentiation of cardiomyocytes to exacerbate heart failure during pressure overload.302 The above studies suggest that heart failure is often accompanied by abnormal Hippo pathway activation.
In addition, the progression of coronary heart diseases and myocardial infarction may be related to changes in the Hippo pathway.296 YAP/TAZ activity is involved in atherogenesis, which is a characteristic of coronary heart disease.303 Interestingly, specific deletion of YAP in fibroblasts can effectively reduce the fibrotic response and improve cardiac function after myocardial infarction.304
The Hippo pathway in pulmonary diseases
The COVID-19 outbreak has resulted in a substantial focus on the relevant mechanisms of the development of lung diseases. As a signalling pathway that could take part in pulmonary development, the Hippo pathway may contribute to pulmonary diseases when it is dysregulated.162,305 Generally, dysregulation of the Hippo pathway frequently occurs in the progression of three pulmonary diseases: pulmonary arterial hypertension, inflammatory pulmonary diseases, and pulmonary fibrosis.
First, Hippo components such as YAP and LATS1 are dysregulated in pulmonary arterial hypertension. The inactivation of Hippo could induce proliferation and suppress apoptosis in pulmonary arterial smooth muscle cells (PASMCs), which is one of the most important factors in vasculature remodelling in pulmonary arterial hypertension.306,307 Interestingly, recent research revealed that MST1/2, a Hippo component that usually plays an antiproliferative role, supports the abnormal proliferation of PASMCs in pulmonary arterial hypertension via forehead homeobox type O and BUB3.308
Second, the Hippo pathway could regulate the inflammatory response in the lung. In a bacterial pneumonia model, the relative levels of p-YAP and p-TAZ were decreased in alveolar epithelial type II cells (AECIIs). YAP/TAZ activation seemed to be a protective reaction. When YAP/TAZ was deleted in AECIIs, inflammation in the lung became more severe.208 Similarly, the deletion of YAP in lung endothelial cells could lead to inflammation in the lung.309 In summary, activation of the Hippo pathway may contribute to pulmonary inflammation. However, the connection between other Hippo components and pulmonary inflammation needs to be further studied.
Third, Hippo can contribute to the progression of fibrotic diseases in the lung. Pulmonary fibrosis is the result of many interstitial pulmonary diseases and is associated with activated fibroblasts and exuberant extracellular matrix deposition. YAP promotes the proliferation and migration of fibroblasts, inducing the production of collagen and inhibiting epithelial cell differentiation, thus contributing to the progression of idiopathic pulmonary fibrosis.310,311
The Hippo pathway in renal diseases
Renal diseases have been recognized as a serious public health burden in the past decade.312 Chronic kidney diseases have a widespread impact and have received much attention. Diabetic and polycystic kidney diseases are two types of chronic kidney diseases.15,313 They are all related to dysregulation of the Hippo pathway.
In diabetic nephropathy, the abnormal proliferation of glomerular mesangial cells is one of the pathological characteristics. The Hippo pathway is inactivated and contributes to abnormal proliferation.314 In addition, in renal enlargement, which is one of the early structural changes in diabetic nephropathy, the Hippo pathway takes part in regulating the proliferation of tubular epithelial cells.315
Except for diabetes, many recent studies have highlighted Hippo-YAP signalling in renal cyst formation to explain the contribution of the Hippo pathway to polycystic kidney diseases, which are inherited disorders mainly caused by PKD1 or PKD2 mutations. It was reported that PKD1 deficiency in mice resulted in YAP and TAZ activation in cystic tubular epithelia. Knockout of YAP and TAZ in the autosomal dominant polycystic kidney disease model significantly suppressed cystogenesis.316,317 In the pkd2 morphants, the Hippo pathway was inactivated as well, resulting in YAP dephosphorylation and nuclear translocation.318
Furthermore, the Hippo pathway could take part in the formation of a fibrotic environment in the kidney. Renal fibrosis is a pathophysiological hallmark of patients with chronic kidney diseases. Ischaemia‒reperfusion (IR) injury is a common model used to study the acute renal injury-chronic kidney disease transition. In the IR injury model, YAP levels were increased along with the progression of renal fibrosis.319 In other renal injury models with renal fibrosis, such as the obstructive, aristolochic acid and diabetic nephropathy models, the expression of TAZ in the tubulointerstitium was elevated.320 In addition, MST1/2 deletion in renal tubule cells caused progressive renal interstitial fibrosis.319–321
The Hippo pathway in central nervous system disorders
Central nervous system disorders are traditionally classified as early-onset neurodevelopmental and late-onset neurodegenerative diseases.322 Hippo pathway dysregulation is closely associated with neurodegenerative diseases.323
In neurodegenerative diseases such as Alzheimer’s disease (AD), amyotrophic lateral sclerosis (ALS) and Huntington’s disease (HD), the main characteristic is the abnormal death of functional neural cells, which could be mediated by the Hippo pathway.324 In AD, the Hippo pathway seems to be activated. One of the causes of AD is the accumulation of amyloid-beta 42 peptides (Aβ42). The activated Hippo pathway could contribute to Aβ42-induced neural cell death.325 Additionally, in Fused in Sarcoma (FUS)-mediated ALS, activated Hippo participated in neuronal cell death by further activating c-JUN amino-terminal kinase (JNK). Cell death could be rescued by downregulating the Hippo pathway.326 In SOD1(G93A) mice, which are a commonly used mouse ALS model, genetic deletion of MST1 could improve spinal cord motor neuron viability and decrease mortality.327 Finally, in HD mice, deficiency in TEAD/YAP-dependent transcription could lead to necrotic cell death.328 In addition, LATS was activated in the brains of patients with HD.329
The Hippo pathway in hepatic diseases
The Hippo pathway not only affects the physiological regeneration of the liver but also impacts the progression of liver disease. Among the liver diseases that the Hippo pathway can influence, hepatitis and liver fibrosis are two of the most well-documented examples.17,200,330
Alcoholic hepatitis (AH) and nonalcoholic steatohepatitis (NASH) are two kinds of hepatitis that can be regulated by the Hippo pathway. In a mouse model of AH,331 YAP levels were elevated in hepatocytes, while in liver samples from human patients with AH,332 YAP1 mRNA was increased, and the active form of MST1 was decreased, indicating low Hippo pathway activity in AH livers. Similarly, the levels of TAZ and YAP were elevated in mice and human patients with NASH.333 One of the underlying mechanisms by which YAP/TAZ is increased in NASH may be related to the suppression of TAZ degradation mediated by β-TrCP-mediated ubiquitination and degradation.334
Hepatic fibrosis results from chronic liver damage, which can be caused by NASH, alcohol abuse or hepatitis virus infection.335 Carbon tetrachloride (CCl4) is a hepatotoxin that is widely used to establish hepatic fibrosis animal models.336 In hepatic fibrosis caused by CCl4 injection, YAP was increased in the nucleus and cytoplasm in hepatocytes and biliary cells from fibrotic livers. After YAP deletion, the expression of Col1a1 was reduced, suggesting the suppression of fibrogenesis.337 Similarly, Jie et al.338 found that a dopamine receptor D2 antagonist, which could block YAP in macrophages, had the potential to attenuate CCl4-induced liver fibrosis. However, in the IR injury model, YAP plays a protective role against IR stress and decreases IR-induced liver fibrosis.339 Therefore, YAP has a complex role in liver fibrosis that depends on cell type and context.
In terms of the underlying mechanism, the Hippo pathway could contribute to hepatitis and hepatic fibrosis by regulating the activation of hepatic stellate cells. Hepatic stellate cells are important hepatic cells that can secrete various inflammatory molecules and extracellular matrix components to aid the progression of hepatic inflammation and fibrosis.340,341 The effect of YAP/TAZ on the activation of hepatic stellate cells has been verified in multiple studies, supporting the importance of the Hippo pathway in the progression of hepatic inflammation and hepatic fibrosis.333,337,342
The Hippo pathway in immune dysfunction
The immune system consists of two parts: the innate immune system and the adaptive immune system. In the past few years, it was observed that dysregulation of the Hippo pathway can influence both innate and adaptive immunity.18
Innate immunity is the first line of defence that protects the body from infection in a nonspecific way, and the Type I interferon (IFN) response, which is known as IFNα and IFNβ, is an essential defence.343,344 YAP negatively regulates IFN-β signalling. Mice with YAP deficiency showed increased IFN-β levels compared to control mice after being infected with vesicular stomatitis virus and herpes simplex virus type 1 (HSV-1), suggesting enhanced innate immunity. Importantly, YAP deficiency reduced the mortality of mice after HSV-1 challenge.345 In contrast, LATS2 could support innate immunity by increasing INF-β expression after human immunodeficiency virus-1 infection in vitro, and the loss of LATS2 impaired the innate immune response.346 Recently, LATS1 was shown to be essential in regulating the activity of type I IFN signalling.347
Furthermore, the Hippo pathway is important for the function of immune cells that take part in adaptive immunity. First, the Hippo component Mst1/2 could affect the proliferation of naïve T cells and the number of peripheral T cells. Although Mst1 deficiency did not change the process of T-cell development, it could decrease the thymic egress of T cells and increase the proliferation of naïve T cells. Double knockout of Mst1 and Mst2 reduced peripheral T cells, while the deletion of Mst2 alone did not significantly change peripheral T cell numbers.348,349 Besides, the Mst1 deficiency was shown to decrease the numbers of marginal zone B cells and memory B cells.350,351
In addition, among the immune cells that can be regulated by the Hippo pathway, Treg cells and Th17 cells are worthy of attention because of their close connection to autoimmune diseases.352,353 It was reported that Mst1-Mst2 was essential for maintaining the Treg pool, while TAZ contributed to the production of Th17 cells and the function of Tregs. Deletion of Mst1-Mst2 led to autoimmune diseases. However, TAZ knockout made the mice resistant to autoimmune encephalomyelitis.354,355 In the clinic, autoimmune manifestations were found in Mst1-deficient patients as well.356
Therapeutic targeting of the Hippo pathway
The close connection between the Hippo pathway and various diseases indicates that the Hippo pathway is an appealing therapeutic target. Until now, no drug specifically targeting the Hippo pathway has been developed for clinical use, likely due to the relatively short history of this pathway. However, potential Hippo-targeted drugs have been widely investigated in both preclinical (Table 1) and clinical trials (Table 2).357,358 At present, the development of potential drugs mainly focuses on three aspects of the Hippo pathway, including Hippo core kinase activity/expression, downstream YAP/TAZ expression levels and YAP/TAZ-TEAD interactions.7 The Hippo core kinases inhibit YAP/TAZ to control its location and subsequently influence the expression of Hippo target genes.114 Thus, inhibitors of Hippo core kinases can be readily developed. However, inhibiting Hippo core kinases can result in YAP/TAZ activity, which is often associated with pathogenesis, particularly cancer. This could be a concern, although Hippo core kinase inhibitors may promote regeneration and wound healing. Furthermore, when YAP/TAZ translocates into the nucleus, the transcription and expression of Hippo-related genes depend on the interaction between YAP/TAZ and TEAD1-4.148 YAP-TEAD transcriptional activity can be suppressed by reducing the expression of YAP/TAZ, disrupting the YAP-TEAD interaction or inhibiting TEAD activity, which makes these potential targets interfere with the Hippo pathway.
Table 1.
The potential drugs targeting Hippo pathway in preclinical trials
| Mechanism | Drugs | Structure/sequence | Indications | |
|---|---|---|---|---|
| YAP/TAZ nucleus/cytoplasm location | MSTs kinase activity inhibition | XMU-MP-1 | 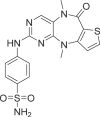 |
Chronic and acute liver injury Protest cancer Breast cancer Autoimmune encephalomyelitis Cardiac hypertrophy |
| SBP-3264 | 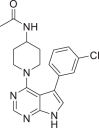 |
Acute myeloid leukaemia | ||
| MSTs kinase expression inhibition | MST1/2-siRNA |
MST1 (5’–3’ sense GAGUGUCAAUAUUGCGAGAtt) MST2 (5’–3’ sense CAAGAGUCAUGAAAAUUGUtt) |
Deficiency of liver regeneration | |
| LATs kinase activity inhibition | TRULI | 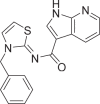 |
No certain indication so far | |
| Sav kinase expression inhibition | Sav-shRNA | Not post |
Myocardial infarction Ischaemic heart failure |
|
| YAP-TEAD transcriptional activity regulation | YAP/TAZ expression inhibition | CA3 | 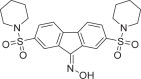 |
Oesophageal adenocarcinoma Osteosarcoma tumour |
| YAP-siRNA | Not post |
Glioblastoma Hepatocellular carcinoma Posterior segment neovascularization-related ocular diseases |
||
| YAP-shRNA | Not post | Lung fibrosis | ||
| YAP-TEAD interaction inhibition | verteporfin | 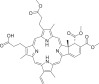 |
Glioblastoma Breast cancer Hepatocellular carcinoma Renal interstitial fibrogenesis Glaucoma |
|
| VGLL4-mimiking peptide | SVDDHFAKSLGDTWLQIGGSGNPK- TANVPQTVPMRLRKLPDSFFKPPE |
Gastric cancer Colorectal cancer |
||
| TEAD palmitoylation inhibition | Flufenamic acid derivative | 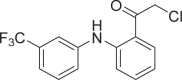 |
Glioblastoma (in vitro) | |
Table 2.
The drugs targeting Hippo pathway in clinical trials
| Mechanism | Name (sponsor) | Phase | Indications | ClinicalTrials.gov Identifier |
|---|---|---|---|---|
| TEAD palmitoylation inhibition | VT3989 (Vivace Therapeutics) | Phase 1 |
Solid Tumour Mesothelioma |
NCT04665206 |
| IK-930 (Ikena Oncology) | Phase 1 |
Solid Tumours Mesothelioma Epithelioid Hemangioendothelioma NF2 Deficiency YAP1 or TAZ Gene Fusions |
NCT05228015 | |
| YAP antisense oligonucleotide | ION537 (Ionis Pharmaceuticals) | Phase 1 | Advanced solid tumours | NCT04659096 |
| Not been disclosed | IAG933 (Novartis) | Phase 1 | Mesothelioma | NCT04857372 |
Hippo core kinase inhibition
Hippo core kinases can be manipulated by kinase inhibition or altering protein expression. MST kinase activity inhibitors are the most common Hippo core kinase activity inhibitors in preclinical studies. In particular, XMU-MP-1, an MST1/2 inhibitor identified by Fan et al.201 has been studied extensively in a variety of diseases. The initial effect of XMU-MP-1 was reported in liver repair and regeneration. Treatment with XMU-MP-1 can ameliorate both chronic and acute liver injury in mouse models in vivo.201 Combined with nanotechnology, Liu et al.359 loaded XMU-MP-1 in a novel nanohybrid to optimize efficacy. In vivo, the XMU-MP-1-loaded nanohybrid showed a longer inhibitory effect on p-YAP and better efficacy in acute liver failure than the free drug. In addition, in cancers such as prostate cancer and breast cancer, MST frequently plays a protumorigenic role. It was reported that XMU-MP-1 may have potential in treating prostate and breast cancer because XMU-MP-1 can inhibit proliferation in a variety of prostate and breast cancer cell lines.360 Moreover, in other mouse models, such as autoimmune encephalomyelitis and cardiac hypertrophy, XMU-MP-1 could relieve these conditions.361,362 However, XMU-MP-1 is not the most ideal inhibitor of MSTs because of its off-target activity.201 Many other MST inhibitors have been identified. Among them, SBP-3264, an MST1/2 small molecule inhibitor that was designed recently, could be used to treat acute myeloid leukaemia.360,363 In addition to MST inhibitors, Hippo core kinase inhibitors have not been extensively examined. Kastan et al.364 identified a potential inhibitor of LATS that was initially named TRULI. However, more details, including the crystallographic information of the binding site and the efficacy of the inhibitor in treating certain diseases, require further investigation.
Experiments to reduce Hippo core components rely on gene knockout/knockdown. The downregulation of multiple Hippo kinases has the potential to treat diseases. For example, it was reported that knocking down Sav significantly ameliorated heart failure.365 Consistently, studies in mice with ischaemic heart failure showed improved heart function after Sav knockdown.301 In partial hepatectomy mouse models, siRNA-mediated knockdown of MST1/2 efficiently induced liver regeneration.330
Inhibition of YAP/TAZ expression/activity
Modulation of YAP/TAZ protein levels or activity mainly involves two approaches in preclinical and clinical studies: pharmacological inhibition and genetic inhibition. CA3 is the representative pharmacological inhibitor of YAP expression that was identified through chemical library screening; however, the detailed mechanism has not been studied thoroughly.366 CA3 treatment reduces the growth of oesophageal adenocarcinoma and osteosarcoma.366,367
Genetic inhibition is another commonly used approach to decrease the expression of YAP/TAZ. siRNA therapy is an alternative to small-molecule inhibitors and has more specificity.368 The efficacy of YAP/TAZ-based siRNA therapy has been verified in a variety of animal models. D/R@Ang2-Lip+Au, a doxorubicin- and YAP-siRNA-loaded cationic liposome, was able to effectively inhibit glioblastoma.369 In addition, another YAP-siRNA-lipid nanoparticle repressed hepatocellular carcinoma in a mouse model.370 In addition to cancer treatment, YAP-siRNA was effective in treating posterior segment neovascularization-related ocular diseases.371 In addition to siRNA, shRNA is another common tool for genetic inhibition. Bleomycin-induced pulmonary fibrosis was attenuated when YAP was downregulated by AAV5-sh-YAP1 and AAV-miR-15a treatment.310 This proven efficacy and unique advantages make genetic inhibitors of YAP/TAZ an exciting potential development prospect. Most notably, thus far, ION537, an antisense medicine targeting YAP1, has been evaluated in phase I clinical trials. This is a great breakthrough for the development of a Hippo-targeted drug (NCT04659096).372
As awareness of the Hippo pathway continues to improve, new ways to inhibit YAP/TAZ have been discovered recently. SuperHippo, a designed WWC-derived protein, was shown to inhibit YAP/TAZ activity by inducing the phosphorylation of LATS1/2 due to the requirement of the WWC protein in the activation of LATS1/2.31
Inhibition of TEAD and/or the YAP-TEAD interaction
YAP-TEAD interaction inhibitors currently offer the diverse potential to target the Hippo pathway. Liu-Chittenden et al. found that verteporfin (VP) could bind to YAP and thereby disrupt its interaction with TEAD.373 In subsequent studies, VP was widely used to treat various diseases, including cancers,374–378 fibrotic diseases379,380 and glaucoma.381 Moreover, as a recognized YAP-TEAD interaction or YAP inhibitor, VP has been frequently used in studies of Hippo-related mechanisms.382,383 However, similar to other potential Hippo-targeted small molecule inhibitors, VP was shown to have off-target and YAP-independent toxic effects.384
Other widely discussed Hippo-targeted YAP-TEAD inhibitors are VGLL4 peptide mimetics. Based on the mechanism by which VGLL4 can bind to TEAD and compete with YAP/TAZ,385,386 VGLL4 peptide mimetics were designed to disrupt the YAP–TEAD interaction. Studies of their efficacy are mainly related to cancer treatment. For example, Super-TDU was designed by Jiao et al. to treat gastric cancer in mouse models. After Super-TD treatment, YAP target genes were suppressed, and the tumour was markedly remitted.44 In another study, Super-TDU was used to treat colorectal cancer. Similarly, tumour growth was significantly suppressed. Overall, VGLL4 peptide mimetics may be potential Hippo-targeted cancer drugs.387
With further exploration of the YAP–TEAD complex structure, the palmitoylation pocket of TEAD has become a new target for inhibiting the transcriptional activity of YAP–TEAD. Palmitoylation is a kind of protein modification that changes cysteine thiols of the substrate protein to thioesters with a palmitoyl group.388 In TEAD, it was previously found that the palmitate chain was inserted into a hydrophobic pocket of TEAD, and palmitoylation played an important role in the binding between TEAD and YAP/TAZ.389 The TEAD transcription factor is very unique in these properties and is not shared by any other transcription factors that normally are very difficult to target. In contrast, due to palmitoylation, TEAD is rather easy to target. Thus, there have been pharmaceutical efforts to develop TEAD inhibitors. Chloromethyl ketone 2, a derivative of FA that was identified in 2019, has been proven to disrupt TEAD4 binding to YAP1 by binding to the TEAD4 palmitate pocket. In vitro, the potential for treating glioblastoma has been demonstrated.390 Strikingly, three TEAD palmitoylation inhibitors have been examined in human clinical trials. VT3989 was developed by Vivice Therapeutics (NCT04665206), and the oral inhibitor IK-930 was by Ikena Oncology (NCT05228015).
Conclusions and perspectives
Due to extensive basic research, the key components of the Hippo pathway network, including MST1/2, LATS1/2, MOB1A/B, SAV1, YAP/TAZ, and TEAD1–4, have been defined. Furthermore, other proteins, such as NF2, MAP4Ks, and WWC1/2/3, were revealed as important elements of the Hippo pathway. The main components and regulatory mechanisms of the Hippo pathway are already in place. The discovery of new components may further improve our understanding of the Hippo pathway in the context of many physiological and pathological processes. Additionally, although a number of upstream signals have been shown to influence the activity of the Hippo pathway, the comprehensive and meticulous interpretation of how these upstream cues are transmitted to Hippo components is incompletely understood.
Moreover, since the Hippo pathway is involved in the regulation of many crucial physiological processes, such as the proliferation and differentiation of cells, embryogenesis, and tissue regeneration, it is not surprising that dysregulation of the Hippo pathway is linked to an array of pathological consequences. Therefore, many therapeutic approaches that target Hippo core kinases, YAP/TAZ, or TEAD have been suggested for the treatment of various diseases.
Several strategies may improve the therapeutic value of current Hippo-targeted drug development efforts. First, combining Hippo-targeted therapies with advanced drug delivery systems, such as extracellular vesicles, nanoparticles, and polymeric vectors, could potentially increase the delivery efficiency of drugs. Second, a deeper understanding of the connection between the Hippo pathway and its upstream signals might provide novel perspectives on how the activity of the Hippo pathway can be manipulated. However, it is noteworthy that the Hippo pathway is not only implicated in the development of diseases but plays essential role in the maintenance of physiological homoeostasis. Thus, future drug development needs to examine ways to improve the efficacy of drugs while minimizing their adverse effects on the normal functions of the Hippo pathway.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 82273297). All the figures were created on BioRender.com.
Author contributions
M.Y.F. and Y.H. drafted this paper. Y.H. and T.X.L. generated the figures and tables. K.-L.G. gave valuable and constructive suggestions for the outline and the content of this paper. T.L. and M.L. developed and revised this paper. All authors have read and approved this paper.
Competing interests
K.L.G. is a co-founder of and has equity interest in Vivace Therapeutics. Other authors declare that they have no conflict of interest.
Footnotes
These authors contributed equally: Minyang Fu, Yuan Hu, Tianxia Lan.
Change history
1/4/2024
A Correction to this paper has been published: 10.1038/s41392-023-01682-3
Contributor Information
Ting Luo, Email: luoting@wchscu.cn.
Min Luo, Email: minluo_scu@163.com.
References
- 1.Cheng J, Wang S, Dong Y, Yuan Z. The role and regulatory mechanism of Hippo signaling components in the neuronal system. Front. Immunol. 2020;11:281. doi: 10.3389/fimmu.2020.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kwon Y, et al. The Hippo signaling pathway interactome. Science. 2013;342:737–740. doi: 10.1126/science.1243971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nat. Rev. Cancer. 2013;13:246–257. doi: 10.1038/nrc3458. [DOI] [PubMed] [Google Scholar]
- 4.Yu FX, et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150:780–791. doi: 10.1016/j.cell.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Misra JR, Irvine KD. The Hippo signaling network and its biological functions. Annu. Rev. Genet. 2018;52:65–87. doi: 10.1146/annurev-genet-120417-031621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calses PC, Crawford JJ, Lill JR, Dey A. Hippo pathway in cancer: aberrant regulation and therapeutic opportunities. Trends Cancer. 2019;5:297–307. doi: 10.1016/j.trecan.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Dey A, Varelas X, Guan KL. Targeting the Hippo pathway in cancer, fibrosis, wound healing and regenerative medicine. Nat. Rev. Drug Discov. 2020;19:480–494. doi: 10.1038/s41573-020-0070-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu JY, Lin S, Ye J. YAP and TAZ, the conductors that orchestrate eye development, homeostasis, and disease. J. Cell. Physiol. 2018;234:246–258. doi: 10.1002/jcp.26870. [DOI] [PubMed] [Google Scholar]
- 9.Lee M, Goraya N, Kim S, Cho SH. Hippo-YAP signaling in ocular development and disease. Dev. Dyn. 2018;247:794–806. doi: 10.1002/dvdy.24628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J, Liu S, Heallen T, Martin JF. The Hippo pathway in the heart: pivotal roles in development, disease, and regeneration. Nat. Rev. Cardiol. 2018;15:672–684. doi: 10.1038/s41569-018-0063-3. [DOI] [PubMed] [Google Scholar]
- 11.Austin KM, et al. Molecular mechanisms of arrhythmogenic cardiomyopathy. Nat. Rev. Cardiol. 2019;16:519–537. doi: 10.1038/s41569-019-0200-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun M, et al. New insights into the Hippo/YAP pathway in idiopathic pulmonary fibrosis. Pharm. Res. 2021;169:105635. doi: 10.1016/j.phrs.2021.105635. [DOI] [PubMed] [Google Scholar]
- 13.Chanda D, et al. Developmental pathways in the pathogenesis of lung fibrosis. Mol. Asp. Med. 2019;65:56–69. doi: 10.1016/j.mam.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma S, Guan KL. Polycystic kidney disease: a Hippo connection. Genes Dev. 2018;32:737–739. doi: 10.1101/gad.316570.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bergmann C, et al. Polycystic kidney disease. Nat. Rev. Dis. Prim. 2018;4:50. doi: 10.1038/s41572-018-0047-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Russell JO, Camargo FD. Hippo signalling in the liver: role in development, regeneration and disease. Nat. Rev. Gastroenterol. Hepatol. 2022;19:297–312. doi: 10.1038/s41575-021-00571-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Driskill JH, Pan D. The Hippo pathway in liver homeostasis and pathophysiology. Annu. Rev. Pathol. 2021;16:299–322. doi: 10.1146/annurev-pathol-030420-105050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hong L, et al. Role of Hippo signaling in regulating immunity. Cell. Mol. Immunol. 2018;15:1003–1009. doi: 10.1038/s41423-018-0007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Zhang H, Zhao B. Hippo signaling in the immune system. Trends Biochem. Sci. 2018;43:77–80. doi: 10.1016/j.tibs.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 20.Ma S, Meng Z, Chen R, Guan KL. The Hippo pathway: biology and pathophysiology. Annu. Rev. Biochem. 2019;88:577–604. doi: 10.1146/annurev-biochem-013118-111829. [DOI] [PubMed] [Google Scholar]
- 21.Piccolo S, Dupont S, Cordenonsi M. The biology of YAP/TAZ: hippo signaling and beyond. Physiol. Rev. 2014;94:1287–1312. doi: 10.1152/physrev.00005.2014. [DOI] [PubMed] [Google Scholar]
- 22.Rausch V, Hansen CG. The Hippo pathway, YAP/TAZ, and the plasma membrane. Trends Cell Biol. 2020;30:32–48. doi: 10.1016/j.tcb.2019.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Bae SJ, et al. SAV1 promotes Hippo kinase activation through antagonizing the PP2A phosphatase STRIPAK. Elife. 2017;6:e30278. doi: 10.7554/eLife.30278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ribeiro PS, et al. Combined functional genomic and proteomic approaches identify a PP2A complex as a negative regulator of Hippo signaling. Mol. Cell. 2010;39:521–534. doi: 10.1016/j.molcel.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Zheng Y, Pan D. The Hippo signaling pathway in development and disease. Dev. Cell. 2019;50:264–282. doi: 10.1016/j.devcel.2019.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen R, et al. STRIPAK integrates upstream signals to initiate the Hippo kinase cascade. Nat. Cell Biol. 2019;21:1565–1577. doi: 10.1038/s41556-019-0426-y. [DOI] [PubMed] [Google Scholar]
- 27.Yin F, et al. Spatial organization of Hippo signaling at the plasma membrane mediated by the tumor suppressor Merlin/NF2. Cell. 2013;154:1342–1355. doi: 10.1016/j.cell.2013.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Badouel C, McNeill H. SnapShot: the Hippo signaling pathway. Cell. 2011;145:484–484. doi: 10.1016/j.cell.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 29.Yu FX, Guan KL. The Hippo pathway: regulators and regulations. Genes Dev. 2013;27:355–371. doi: 10.1101/gad.210773.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chan EH, et al. The Ste20-like kinase Mst2 activates the human large tumor suppressor kinase Lats1. Oncogene. 2005;24:2076–2086. doi: 10.1038/sj.onc.1208445. [DOI] [PubMed] [Google Scholar]
- 31.Qi S, et al. WWC proteins mediate LATS1/2 activation by Hippo kinases and imply a tumor suppression strategy. Mol. Cell. 2022;82:1850–1864. doi: 10.1016/j.molcel.2022.03.027. [DOI] [PubMed] [Google Scholar]
- 32.Li Q, et al. The conserved Misshapen–Warts–Yorkie pathway acts in enteroblasts to regulate intestinal stem cells in. Drosoph. Dev. Cell. 2014;31:291–304. doi: 10.1016/j.devcel.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li S, et al. Overlapping functions of the MAP4K family kinases Hppy and Msn in Hippo signaling. Cell Discov. 2015;1:15038. doi: 10.1038/celldisc.2015.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meng Z, et al. MAP4K family kinases act in parallel to MST1/2 to activate LATS1/2 in the Hippo pathway. Nat. Commun. 2015;6:8357. doi: 10.1038/ncomms9357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng Y, et al. Identification of Happyhour/MAP4K as alternative Hpo/Mst-like kinases in the Hippo kinase cascade. Dev. Cell. 2015;34:642–655. doi: 10.1016/j.devcel.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Plouffe SW, et al. Characterization of Hippo pathway components by gene inactivation. Mol. Cell. 2016;64:993–1008. doi: 10.1016/j.molcel.2016.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wei X, Shimizu T, Lai ZC. Mob as tumor suppressor is activated by Hippo kinase for growth inhibition in. Drosoph. EMBO J. 2007;26:1772–1781. doi: 10.1038/sj.emboj.7601630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Praskova M, Xia F, Avruch J. MOBKL1A/MOBKL1B phosphorylation by MST1 and MST2 inhibits cell proliferation. Curr. Biol. 2008;18:311–321. doi: 10.1016/j.cub.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oka T, Mazack V, Sudol M. Mst2 and Lats kinases regulate apoptotic function of Yes kinase-associated protein (YAP) J. Biol. Chem. 2008;283:27534–27546. doi: 10.1074/jbc.M804380200. [DOI] [PubMed] [Google Scholar]
- 40.Hao Y, et al. Tumor suppressor LATS1 is a negative regulator of oncogene YAP. J. Biol. Chem. 2008;283:5496–5509. doi: 10.1074/jbc.M709037200. [DOI] [PubMed] [Google Scholar]
- 41.Moroishi T, et al. The Hippo pathway kinases LATS1/2 suppress cancer immunity. Cell. 2016;167:1525–1539e1517. doi: 10.1016/j.cell.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pocaterra A, Romani P, Dupont S. YAP/TAZ functions and their regulation at a glance. J. Cell Sci. 2020;133:jcs230425. doi: 10.1242/jcs.230425. [DOI] [PubMed] [Google Scholar]
- 43.Ma S, et al. Transcriptional repression of estrogen receptor alpha by YAP reveals the Hippo pathway as therapeutic target for ER(+) breast cancer. Nat. Commun. 2022;13:1061. doi: 10.1038/s41467-022-28691-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiao S, et al. A peptide mimicking VGLL4 function acts as a YAP antagonist therapy against gastric cancer. Cancer Cell. 2014;25:166–180. doi: 10.1016/j.ccr.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 45.Zhang W, et al. VGLL4 functions as a new tumor suppressor in lung cancer by negatively regulating the YAP–TEAD transcriptional complex. Cell Res. 2014;24:331–343. doi: 10.1038/cr.2014.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin KC, Park HW, Guan K-L. Regulation of the Hippo pathway transcription factor TEAD. Trends Biochem. Sci. 2017;42:862–872. doi: 10.1016/j.tibs.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guo T, et al. A novel partner of Scalloped regulates Hippo signaling via antagonizing Scalloped-Yorkie activity. Cell Res. 2013;23:1201–1214. doi: 10.1038/cr.2013.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koontz LM, et al. The Hippo effector Yorkie controls normal tissue growth by antagonizing scalloped-mediated default repression. Dev. Cell. 2013;25:388–401. doi: 10.1016/j.devcel.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maejima, Y., Zablocki, D., Nah, J. & Sadoshima, J. The role of the Hippo pathway in autophagy in the heart. Cardiovasc. Res. cvac014 (2022). [DOI] [PMC free article] [PubMed]
- 50.Tian Q, et al. RICH1 inhibits breast cancer stem cell traits through activating kinases cascade of Hippo signaling by competing with Merlin for binding to Amot-p80. Cell Death Dis. 2022;13:71. doi: 10.1038/s41419-022-04516-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guo Y, et al. Context-dependent transcriptional regulations of YAP/TAZ in cancer. Cancer Lett. 2022;527:164–173. doi: 10.1016/j.canlet.2021.12.019. [DOI] [PubMed] [Google Scholar]
- 52.Mok JW, Choi KW. Modulation of Hippo signaling by Mnat9 N-acetyltransferase for normal growth and tumorigenesis in. Drosoph. Cell Death Dis. 2022;13:101. doi: 10.1038/s41419-022-04532-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cho YS, Jiang J. Hippo-independent regulation of Yki/Yap/Taz: a non-canonical view. Front. Cell Dev. Biol. 2021;9:658481. doi: 10.3389/fcell.2021.658481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stepan J, Anderzhanova E, Gassen NC. Hippo signaling: emerging pathway in stress-related psychiatric disorders? Front. Psychiatry. 2018;9:715. doi: 10.3389/fpsyt.2018.00715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun T, Chi JT. Regulation of ferroptosis in cancer cells by YAP/TAZ and Hippo pathways: the therapeutic implications. Genes Dis. 2021;8:241–249. doi: 10.1016/j.gendis.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reggiani F, Gobbi G, Ciarrocchi A, Sancisi V. YAP and TAZ are not identical twins. Trends Biochem. Sci. 2021;46:154–168. doi: 10.1016/j.tibs.2020.08.012. [DOI] [PubMed] [Google Scholar]
- 57.Abdi K, Kuo CT. Laminating the mammalian cortex during development: cell polarity protein function and Hippo signaling. Genes Dev. 2018;32:740–741. doi: 10.1101/gad.316711.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Buckley CE, St Johnston D. Apical–basal polarity and the control of epithelial form and function. Nat. Rev. Mol. Cell Biol. 2022;23:559–577. doi: 10.1038/s41580-022-00465-y. [DOI] [PubMed] [Google Scholar]
- 59.Simons M, Mlodzik M. Planar cell polarity signaling: from fly development to human disease. Annu. Rev. Genet. 2008;42:517–540. doi: 10.1146/annurev.genet.42.110807.091432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schroeder MC, Halder G. Regulation of the Hippo pathway by cell architecture and mechanical signals. Semin. Cell Dev. Biol. 2012;23:803–811. doi: 10.1016/j.semcdb.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 61.Bretscher A, Edwards K, Fehon RG. ERM proteins and merlin: integrators at the cell cortex. Nat. Rev. Mol. Cell Biol. 2002;3:586–599. doi: 10.1038/nrm882. [DOI] [PubMed] [Google Scholar]
- 62.Su T, Ludwig MZ, Xu J, Fehon RG. Kibra and Merlin activate the Hippo pathway spatially distinct from and independent of expanded. Dev. Cell. 2017;40:478–490e473. doi: 10.1016/j.devcel.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wei Y, et al. NEDD4L-mediated Merlin ubiquitination facilitates Hippo pathway activation. EMBO Rep. 2020;21:e50642–e50642. doi: 10.15252/embr.202050642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang N, et al. The Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Dev. Cell. 2010;19:27–38. doi: 10.1016/j.devcel.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Matsuda T, et al. NF2 activates Hippo signaling and promotes ischemia/reperfusion injury in the heart. Circ. Res. 2016;119:596–606. doi: 10.1161/CIRCRESAHA.116.308586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ding Y, et al. Hippo signaling suppresses tumor cell metastasis via a Yki-Src42A positive feedback loop. Cell Death Dis. 2021;12:1126. doi: 10.1038/s41419-021-04423-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shen H, et al. SCRIB promotes proliferation and metastasis by targeting Hippo/YAP signalling in colorectal cancer. Front. Cell Dev. Biol. 2021;9:656359–656359. doi: 10.3389/fcell.2021.656359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Varelas X, et al. The Crumbs complex couples cell density sensing to Hippo-dependent control of the TGF-β-SMAD pathway. Dev. Cell. 2010;19:831–844. doi: 10.1016/j.devcel.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 69.Wang W, Huang J, Chen J. Angiomotin-like proteins associate with and negatively regulate YAP1. J. Biol. Chem. 2011;286:4364–4370. doi: 10.1074/jbc.C110.205401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hirate Y, et al. Polarity-dependent distribution of angiomotin localizes Hippo signaling in preimplantation embryos. Curr. Biol. 2013;23:1181–1194. doi: 10.1016/j.cub.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mana-Capelli S, McCollum D. Angiomotins stimulate LATS kinase autophosphorylation and act as scaffolds that promote Hippo signaling. J. Biol. Chem. 2018;293:18230–18241. doi: 10.1074/jbc.RA118.004187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Robinson BS, Moberg KH. Cell–cell junctions: α-catenin and E-cadherin help fence in Yap1. Curr. Biol. 2011;21:R890–R892. doi: 10.1016/j.cub.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Silvis MR, et al. α-catenin is a tumor suppressor that controls cell accumulation by regulating the localization and activity of the transcriptional coactivator Yap1. Sci. Signal. 2011;4:ra33. doi: 10.1126/scisignal.2001823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Beutel O, et al. Phase separation of Zonula Occludens proteins drives formation of tight junctions. Cell. 2019;179:923–936. doi: 10.1016/j.cell.2019.10.011. [DOI] [PubMed] [Google Scholar]
- 75.Domínguez-Calderón A, et al. ZO-2 silencing induces renal hypertrophy through a cell cycle mechanism and the activation of YAP and the mTOR pathway. Mol. Biol. Cell. 2016;27:1581–1595. doi: 10.1091/mbc.E15-08-0598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bryant PJ, et al. Mutations at the fat locus interfere with cell proliferation control and epithelial morphogenesis in. Drosoph. Dev. Biol. 1988;129:541–554. doi: 10.1016/0012-1606(88)90399-5. [DOI] [PubMed] [Google Scholar]
- 77.Mahoney PA, et al. The fat tumor suppressor gene in Drosophila encodes a novel member of the cadherin gene superfamily. Cell. 1991;67:853–868. doi: 10.1016/0092-8674(91)90359-7. [DOI] [PubMed] [Google Scholar]
- 78.Sopko R, McNeill H. The skinny on fat: an enormous cadherin that regulates cell adhesion, tissue growth, and planar cell polarity. Curr. Opin. Cell Biol. 2009;21:717–723. doi: 10.1016/j.ceb.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 79.Lawrence PA, Struhl G, Casal J. Do the protocadherins fat and Dachsous link up to determine both planar cell polarity and the dimensions of organs? Nat. Cell Biol. 2008;10:1379–1382. doi: 10.1038/ncb1208-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Irvine KD, Harvey KF. Control of organ growth by patterning and hippo signaling in. Drosoph. Cold Spring Harb. Perspect. Biol. 2015;7:a019224. doi: 10.1101/cshperspect.a019224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pan G, et al. Signal transduction by the fat cytoplasmic domain. Development. 2013;140:831–842. doi: 10.1242/dev.088534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mao Y, et al. Dachs: an unconventional myosin that functions downstream of fat to regulate growth, affinity and gene expression in. Drosoph. Dev. 2006;133:2539–2551. doi: 10.1242/dev.02427. [DOI] [PubMed] [Google Scholar]
- 83.Vrabioiu AM, Struhl G. Fat/Dachsous signaling promotes Drosophila wing growth by regulating the conformational state of the NDR kinase warts. Dev. Cell. 2015;35:737–749. doi: 10.1016/j.devcel.2015.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dupont S, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 85.Dasgupta I, McCollum D. Control of cellular responses to mechanical cues through YAP/TAZ regulation. J. Biol. Chem. 2019;294:17693–17706. doi: 10.1074/jbc.REV119.007963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Meng Z, et al. RAP2 mediates mechanoresponses of the Hippo pathway. Nature. 2018;560:655–660. doi: 10.1038/s41586-018-0444-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chakraborty S, et al. Agrin as a mechanotransduction signal regulating YAP through the Hippo pathway. Cell Rep. 2017;18:2464–2479. doi: 10.1016/j.celrep.2017.02.041. [DOI] [PubMed] [Google Scholar]
- 88.Li Z, et al. Tenascin-C-mediated suppression of extracellular matrix adhesion force promotes entheseal new bone formation through activation of Hippo signalling in ankylosing spondylitis. Ann. Rheum. Dis. 2021;80:891–902. doi: 10.1136/annrheumdis-2021-220002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pereira D, et al. Cell geometry and the cytoskeleton impact the nucleo-cytoplasmic localisation of the SMYD3 methyltransferase. Sci. Rep. 2020;10:20598. doi: 10.1038/s41598-020-75833-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wada K-I, et al. Hippo pathway regulation by cell morphology and stress fibers. Development. 2011;138:3907–3914. doi: 10.1242/dev.070987. [DOI] [PubMed] [Google Scholar]
- 91.Roux E, Bougaran P, Dufourcq P, Couffinhal T. Fluid shear stress sensing by the endothelial layer. Front. Physiol. 2020;11:861. doi: 10.3389/fphys.2020.00861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Reichenbach M, et al. Differential impact of fluid shear stress and YAP/TAZ on BMP/TGF-β Induced osteogenic target genes. Adv. Biol. 2021;5:2000051. doi: 10.1002/adbi.202000051. [DOI] [PubMed] [Google Scholar]
- 93.Lv H, Ai D. Hippo/yes-associated protein signaling functions as a mechanotransducer in regulating vascular homeostasis. J. Mol. Cell. Cardiol. 2022;162:158–165. doi: 10.1016/j.yjmcc.2021.09.007. [DOI] [PubMed] [Google Scholar]
- 94.Zhong W, et al. Mesenchymal stem cell and chondrocyte fates in a multishear microdevice are regulated by Yes-associated protein. Stem Cells Dev. 2013;22:2083–2093. doi: 10.1089/scd.2012.0685. [DOI] [PubMed] [Google Scholar]
- 95.Zhao B, et al. Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis. Genes Dev. 2012;26:54–68. doi: 10.1101/gad.173435.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ibar C, et al. Tension-dependent regulation of mammalian Hippo signaling through LIMD1. J. Cell Sci. 2018;131:jcs214700. doi: 10.1242/jcs.214700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rauskolb C, et al. Cytoskeletal tension inhibits Hippo signaling through an Ajuba–Warts complex. Cell. 2014;158:143–156. doi: 10.1016/j.cell.2014.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li J, et al. Zipper-interacting protein kinase promotes epithelial-mesenchymal transition, invasion and metastasis through AKT and NF-kB signaling and is associated with metastasis and poor prognosis in gastric cancer patients. Oncotarget. 2015;6:8323–8338. doi: 10.18632/oncotarget.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Short B. Integrin signaling tranquilizes Hippo. J. Cell Biol. 2015;210:364–364. [Google Scholar]
- 100.Zhu B, et al. Piezo 1 activation facilitates cholangiocarcinoma metastasis via Hippo/YAP signaling axis. Mol. Ther. Nucleic Acids. 2021;24:241–252. doi: 10.1016/j.omtn.2021.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jiang C, et al. Mechanochemical control of epidermal stem cell divisions by B-plexins. Nat. Commun. 2021;12:1308–1308. doi: 10.1038/s41467-021-21513-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dobrokhotov O, Samsonov M, Sokabe M, Hirata H. Mechanoregulation and pathology of YAP/TAZ via Hippo and non-Hippo mechanisms. Clin. Transl. Med. 2018;7:23. doi: 10.1186/s40169-018-0202-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dupont S. Regulation of YAP/TAZ activity by mechanical cues: an experimental overview. Methods Mol. Biol. 2019;1893:183–202. doi: 10.1007/978-1-4939-8910-2_15. [DOI] [PubMed] [Google Scholar]
- 104.Gumbiner BM, Kim N-G. The Hippo-YAP signaling pathway and contact inhibition of growth. J. Cell Sci. 2014;127:709–717. doi: 10.1242/jcs.140103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhao B, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sharif GM, Wellstein A. Cell density regulates cancer metastasis via the Hippo pathway. Future Oncol. 2015;11:3253–3260. doi: 10.2217/fon.15.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Paul A, et al. Cell adhesion molecule KIRREL1 is a feedback regulator of Hippo signaling recruiting SAV1 to cell-cell contact sites. Nat. Commun. 2022;13:930. doi: 10.1038/s41467-022-28567-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gu Y, et al. Transmembrane protein KIRREL1 regulates Hippo signaling via a feedback loop and represents a therapeutic target in YAP/TAZ-active cancers. Cell Rep. 2022;40:111296. doi: 10.1016/j.celrep.2022.111296. [DOI] [PubMed] [Google Scholar]
- 109.Wang C, et al. Integrated screens uncover a cell surface tumor suppressor gene KIRREL involved in Hippo pathway. Proc. Natl Acad. Sci. USA. 2022;119:e2121779119. doi: 10.1073/pnas.2121779119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kim NG, Gumbiner BM. Cell contact and Nf2/Merlin-dependent regulation of TEAD palmitoylation and activity. Proc. Natl Acad. Sci. USA. 2019;116:9877–9882. doi: 10.1073/pnas.1819400116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wu J, et al. Intercellular interaction dictates cancer cell ferroptosis via NF2-YAP signalling. Nature. 2019;572:402–406. doi: 10.1038/s41586-019-1426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shalhout SZ, et al. YAP-dependent proliferation by a small molecule targeting annexin A2. Nat. Chem. Biol. 2021;17:767–775. doi: 10.1038/s41589-021-00755-0. [DOI] [PubMed] [Google Scholar]
- 113.Ooki T, et al. High-molecular-weight hyaluronan is a Hippo Pathway ligand directing cell density-dependent growth inhibition via PAR1b. Dev. Cell. 2019;49:590–604. doi: 10.1016/j.devcel.2019.04.018. [DOI] [PubMed] [Google Scholar]
- 114.Meng Z, Moroishi T, Guan K-L. Mechanisms of Hippo pathway regulation. Genes Dev. 2016;30:1–17. doi: 10.1101/gad.274027.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gurevich EV, Gainetdinov RR, Gurevich VV. G protein-coupled receptor kinases as regulators of dopamine receptor functions. Pharm. Res. 2016;111:1–16. doi: 10.1016/j.phrs.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Koo JH, Guan KL. Interplay between YAP/TAZ and metabolism. Cell Metab. 2018;28:196–206. doi: 10.1016/j.cmet.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 117.Choi KM, et al. GPCR-mediated YAP/TAZ inactivation in fibroblasts via EPAC1/2, RAP2C, and MAP4K7. J. Cell Physiol. 2021;236:7759–7774. doi: 10.1002/jcp.30459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zindel D, et al. G protein-coupled receptors can control the Hippo/YAP pathway through Gq signaling. FASEB J. 2021;35:e21668. doi: 10.1096/fj.202002159R. [DOI] [PubMed] [Google Scholar]
- 119.Miller E, et al. Identification of serum-derived sphingosine-1-phosphate as a small molecule regulator of YAP. Chem. Biol. 2012;19:955–962. doi: 10.1016/j.chembiol.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 120.Zmajkovicova K, et al. GPCR-induced YAP activation sensitizes fibroblasts to profibrotic activity of TGFβ1. PLoS ONE. 2020;15:e0228195–e0228195. doi: 10.1371/journal.pone.0228195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhou X, et al. Estrogen regulates Hippo signaling via GPER in breast cancer. J. Clin. Investig. 2015;125:2123–2135. doi: 10.1172/JCI79573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang Z, et al. Endothelin promotes colorectal tumorigenesis by activating YAP/TAZ. Cancer Res. 2017;77:2413–2423. doi: 10.1158/0008-5472.CAN-16-3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Uemura H, et al. Renin-angiotensin system is an important factor in hormone refractory prostate cancer. Prostate. 2006;66:822–830. doi: 10.1002/pros.20407. [DOI] [PubMed] [Google Scholar]
- 124.Kim HB, et al. Prostaglandin E(2) activates YAP and a positive-signaling loop to promote colon regeneration after colitis but also carcinogenesis in mice. Gastroenterology. 2017;152:616–630. doi: 10.1053/j.gastro.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gong R, et al. Opposing roles of conventional and novel PKC isoforms in Hippo-YAP pathway regulation. Cell Res. 2015;25:985–988. doi: 10.1038/cr.2015.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yu Z, et al. IL-17A promotes psoriasis-associated keratinocyte proliferation through ACT1-dependent activation of YAP–AREG axis. J. Investig. Dermatol. 2022;142:2343–2352. doi: 10.1016/j.jid.2022.02.016. [DOI] [PubMed] [Google Scholar]
- 127.Sorrentino G, et al. Glucocorticoid receptor signalling activates YAP in breast cancer. Nat. Commun. 2017;8:14073. doi: 10.1038/ncomms14073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Miranda MZ, et al. TGF-β1 regulates the expression and transcriptional activity of TAZ protein via a Smad3-independent, myocardin-related transcription factor-mediated mechanism. J. Biol. Chem. 2017;292:14902–14920. doi: 10.1074/jbc.M117.780502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yao M, et al. BMP2-SMAD signaling represses the proliferation of embryonic neural stem cells through YAP. J. Neurosci. 2014;34:12039–12048. doi: 10.1523/JNEUROSCI.0486-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Taniguchi K, et al. A gp130-Src-YAP module links inflammation to epithelial regeneration. Nature. 2015;519:57–62. doi: 10.1038/nature14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhou X, et al. Regulation of Hippo-YAP signaling by insulin-like growth factor-1 receptor in the tumorigenesis of diffuse large B-cell lymphoma. J. Hematol. Oncol. 2020;13:77. doi: 10.1186/s13045-020-00906-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ando T, et al. EGFR regulates the Hippo pathway by promoting the tyrosine phosphorylation of MOB1. Commun. Biol. 2021;4:1237. doi: 10.1038/s42003-021-02744-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang X, et al. YAP/TAZ orchestrate VEGF signaling during developmental angiogenesis. Dev. Cell. 2017;42:462–478e467. doi: 10.1016/j.devcel.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 134.Hariharan IK. Energy stress tames the Hippo pathway. Nat. Cell Biol. 2015;17:362–363. doi: 10.1038/ncb3141. [DOI] [PubMed] [Google Scholar]
- 135.Mao B, Gao Y, Bai Y, Yuan Z. Hippo signaling in stress response and homeostasis maintenance. Acta Biochim. Biophys. Sin. 2014;47:2–9. doi: 10.1093/abbs/gmu109. [DOI] [PubMed] [Google Scholar]
- 136.Zeybek DN, Baysal E, Bozdemir O, Buber E. Hippo signaling: a stress response pathway in stem cells. Curr. Stem Cell Res. Ther. 2021;16:824–839. doi: 10.2174/1574888X16666210712100002. [DOI] [PubMed] [Google Scholar]
- 137.Yan L, Cai Q, Xu Y. Hypoxic conditions differentially regulate TAZ and YAP in cancer cells. Arch. Biochem. Biophys. 2014;562:31–36. doi: 10.1016/j.abb.2014.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ma B, et al. Zyxin–Siah2–Lats2 axis mediates cooperation between Hippo and TGF-β signalling pathways. Nat. Commun. 2016;7:11123–11123. doi: 10.1038/ncomms11123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ma B, et al. Hypoxia regulates Hippo signalling through the SIAH2 ubiquitin E3 ligase. Nat. Cell Biol. 2015;17:95–103. doi: 10.1038/ncb3073. [DOI] [PubMed] [Google Scholar]
- 140.Tao EW, et al. A specific tRNA half, 5’tiRNA-His-GTG, responds to hypoxia via the HIF1α/ANG axis and promotes colorectal cancer progression by regulating LATS2. J. Exp. Clin. Cancer Res. 2021;40:67. doi: 10.1186/s13046-021-01836-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Howell SH. Endoplasmic reticulum stress responses in plants. Annu. Rev. Plant Biol. 2013;64:477–499. doi: 10.1146/annurev-arplant-050312-120053. [DOI] [PubMed] [Google Scholar]
- 142.Wu H, et al. Integration of Hippo signalling and the unfolded protein response to restrain liver overgrowth and tumorigenesis. Nat. Commun. 2015;6:6239. doi: 10.1038/ncomms7239. [DOI] [PubMed] [Google Scholar]
- 143.Mo JS, et al. Cellular energy stress induces AMPK-mediated regulation of YAP and the Hippo pathway. Nat. Cell Biol. 2015;17:500–510. doi: 10.1038/ncb3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang W, et al. AMPK modulates Hippo pathway activity to regulate energy homeostasis. Nat. Cell Biol. 2015;17:490–499. doi: 10.1038/ncb3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hong AW, et al. Osmotic stress-induced phosphorylation by NLK at Ser128 activates YAP. EMBO Rep. 2017;18:72–86. doi: 10.15252/embr.201642681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hong AW, et al. Critical roles of phosphoinositides and NF2 in Hippo pathway regulation. Genes Dev. 2020;34:511–525. doi: 10.1101/gad.333435.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Luo M, et al. Heat stress activates YAP/TAZ to induce the heat shock transcriptome. Nat. Cell Biol. 2020;22:1447–1459. doi: 10.1038/s41556-020-00602-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhao B, et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008;22:1962–1971. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Neininger AC, Dai X, Liu Q, Burnette DT. The Hippo pathway regulates density-dependent proliferation of iPSC-derived cardiac myocytes. Sci. Rep. 2021;11:17759. doi: 10.1038/s41598-021-97133-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Heallen T, et al. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science. 2011;332:458–461. doi: 10.1126/science.1199010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lin Z, et al. Pi3kcb links Hippo-YAP and PI3K-AKT signaling pathways to promote cardiomyocyte proliferation and survival. Circ. Res. 2015;116:35–45. doi: 10.1161/CIRCRESAHA.115.304457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Morikawa Y, et al. Dystrophin–glycoprotein complex sequesters Yap to inhibit cardiomyocyte proliferation. Nature. 2017;547:227–231. doi: 10.1038/nature22979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Diez-Cuñado M, et al. miRNAs that induce human cardiomyocyte proliferation converge on the Hippo pathway. Cell Rep. 2018;23:2168–2174. doi: 10.1016/j.celrep.2018.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yuan Y, et al. YAP1/TAZ-TEAD transcriptional networks maintain skin homeostasis by regulating cell proliferation and limiting KLF4 activity. Nat. Commun. 2020;11:1472. doi: 10.1038/s41467-020-15301-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Corley SM, et al. Plau and Tgfbr3 are YAP-regulated genes that promote keratinocyte proliferation. Cell Death Dis. 2018;9:1106. doi: 10.1038/s41419-018-1141-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hasegawa K, et al. YAP signaling induces PIEZO1 to promote oral squamous cell carcinoma cell proliferation. J. Pathol. 2021;253:80–93. doi: 10.1002/path.5553. [DOI] [PubMed] [Google Scholar]
- 157.Mi W, et al. Geranylgeranylation signals to the Hippo pathway for breast cancer cell proliferation and migration. Oncogene. 2015;34:3095–3106. doi: 10.1038/onc.2014.251. [DOI] [PubMed] [Google Scholar]
- 158.Deng Y, et al. A reciprocal regulatory loop between TAZ/YAP and G-protein Gαs regulates Schwann cell proliferation and myelination. Nat. Commun. 2017;8:15161. doi: 10.1038/ncomms15161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Schlegelmilch K, et al. Yap1 acts downstream of α-catenin to control epidermal proliferation. Cell. 2011;144:782–795. doi: 10.1016/j.cell.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yi C, et al. The p130 isoform of angiomotin is required for Yap-mediated hepatic epithelial cell proliferation and tumorigenesis. Sci. Signal. 2013;6:ra77–ra77. doi: 10.1126/scisignal.2004060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hu C, et al. The Hippo–YAP pathway regulates the proliferation of alveolar epithelial progenitors after acute lung injury. Cell Biol. Int. 2019;43:1174–1183. doi: 10.1002/cbin.11098. [DOI] [PubMed] [Google Scholar]
- 162.Lin C, et al. YAP is essential for mechanical force production and epithelial cell proliferation during lung branching morphogenesis. Elife. 2017;6:e21130. doi: 10.7554/eLife.21130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gao T, et al. Hippo signaling regulates differentiation and maintenance in the exocrine pancreas. Gastroenterology. 2013;144:1543–1553e1541. doi: 10.1053/j.gastro.2013.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lange AW, et al. Hippo/Yap signaling controls epithelial progenitor cell proliferation and differentiation in the embryonic and adult lung. J. Mol. Cell Biol. 2015;7:35–47. doi: 10.1093/jmcb/mju046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lin C, Yao E, Chuang PT. A conserved MST1/2–YAP axis mediates Hippo signaling during lung growth. Dev. Biol. 2015;403:101–113. doi: 10.1016/j.ydbio.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Szymaniak AD, Mahoney JE, Cardoso WV, Varelas X. Crumbs3-mediated polarity directs airway epithelial cell fate through the Hippo pathway effector Yap. Dev. Cell. 2015;34:283–296. doi: 10.1016/j.devcel.2015.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhao R, et al. Yap tunes airway epithelial size and architecture by regulating the identity, maintenance, and self-renewal of stem cells. Dev. Cell. 2014;30:151–165. doi: 10.1016/j.devcel.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Nantie LB, et al. Lats1/2 inactivation reveals Hippo function in alveolar type I cell differentiation during lung transition to air breathing. Development. 2018;145:dev163105. doi: 10.1242/dev.163105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Jia X, et al. YAP and Wnt3a independently promote AECIIs proliferation and differentiation by increasing nuclear β-catenin expression in experimental bronchopulmonary dysplasia. Int. J. Mol. Med. 2021;47:195–206. doi: 10.3892/ijmm.2020.4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chen TH, et al. YAP promotes myogenic differentiation via the MEK5–ERK5 pathway. FASEB J. 2017;31:2963–2972. doi: 10.1096/fj.201601090R. [DOI] [PubMed] [Google Scholar]
- 171.Judson RN, et al. The Hippo pathway member Yap plays a key role in influencing fate decisions in muscle satellite cells. J. Cell Sci. 2012;125:6009–6019. doi: 10.1242/jcs.109546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wang L, et al. YAP/TAZ are required to suppress osteogenic differentiation of vascular smooth muscle cells. iScience. 2020;23:101860. doi: 10.1016/j.isci.2020.101860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Chen Q, et al. A temporal requirement for Hippo signaling in mammary gland differentiation, growth, and tumorigenesis. Genes Dev. 2014;28:432–437. doi: 10.1101/gad.233676.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.McNeill H, Reginensi A. Lats1/2 regulate Yap/Taz to control nephron progenitor epithelialization and inhibit myofibroblast formation. J. Am. Soc. Nephrol. 2017;28:852–861. doi: 10.1681/ASN.2016060611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Fallah S, Beaulieu JF. Src family kinases inhibit differentiation of intestinal epithelial cells through the Hippo effector YAP1. Biol. Open. 2021;10:bio058904. doi: 10.1242/bio.058904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Yimlamai D, et al. Hippo pathway activity influences liver cell fate. Cell. 2014;157:1324–1338. doi: 10.1016/j.cell.2014.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yi J, et al. Large tumor suppressor homologs 1 and 2 regulate mouse liver progenitor cell proliferation and maturation through antagonism of the coactivators YAP and TAZ. Hepatology. 2016;64:1757–1772. doi: 10.1002/hep.28768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wu Z, Guan KL. Hippo signaling in embryogenesis and development. Trends Biochem. Sci. 2021;46:51–63. doi: 10.1016/j.tibs.2020.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Nishioka N, et al. The Hippo signaling pathway components Lats and Yap pattern Tead4 activity to distinguish mouse trophectoderm from inner cell mass. Dev. Cell. 2009;16:398–410. doi: 10.1016/j.devcel.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 180.Ralston A, et al. Gata3 regulates trophoblast development downstream of Tead4 and in parallel to Cdx2. Development. 2010;137:395–403. doi: 10.1242/dev.038828. [DOI] [PubMed] [Google Scholar]
- 181.Frum T, Watts JL, Ralston A. TEAD4, YAP1 and WWTR1 prevent the premature onset of pluripotency prior to the 16-cell stage. Development. 2019;146:dev179861. doi: 10.1242/dev.179861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lorthongpanich C, et al. Temporal reduction of LATS kinases in the early preimplantation embryo prevents ICM lineage differentiation. Genes Dev. 2013;27:1441–1446. doi: 10.1101/gad.219618.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Cockburn K, Biechele S, Garner J, Rossant J. The Hippo pathway member Nf2 is required for inner cell mass specification. Curr. Biol. 2013;23:1195–1201. doi: 10.1016/j.cub.2013.05.044. [DOI] [PubMed] [Google Scholar]
- 184.Morin-Kensicki EM, et al. Defects in yolk sac vasculogenesis, chorioallantoic fusion, and embryonic axis elongation in mice with targeted disruption of Yap65. Mol. Cell. Biol. 2006;26:77–87. doi: 10.1128/MCB.26.1.77-87.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kim J, et al. YAP/TAZ regulates sprouting angiogenesis and vascular barrier maturation. J. Clin. Investig. 2017;127:3441–3461. doi: 10.1172/JCI93825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.von Gise A, et al. YAP1, the nuclear target of Hippo signaling, stimulates heart growth through cardiomyocyte proliferation but not hypertrophy. Proc. Natl Acad. Sci. USA. 2012;109:2394–2399. doi: 10.1073/pnas.1116136109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Mahoney JE, et al. The hippo pathway effector Yap controls patterning and differentiation of airway epithelial progenitors. Dev. Cell. 2014;30:137–150. doi: 10.1016/j.devcel.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Reginensi A, et al. Yap- and Cdc42-dependent nephrogenesis and morphogenesis during mouse kidney development. PLoS Genet. 2013;9:e1003380. doi: 10.1371/journal.pgen.1003380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Reginensi A, et al. A critical role for NF2 and the Hippo pathway in branching morphogenesis. Nat. Commun. 2016;7:12309. doi: 10.1038/ncomms12309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Moya IM, Halder G. Hippo–YAP/TAZ signalling in organ regeneration and regenerative medicine. Nat. Rev. Mol. Cell Biol. 2019;20:211–226. doi: 10.1038/s41580-018-0086-y. [DOI] [PubMed] [Google Scholar]
- 191.Rodrigues M, Kosaric N, Bonham CA, Gurtner GC. Wound healing: a cellular perspective. Physiol. Rev. 2019;99:665–706. doi: 10.1152/physrev.00067.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Wang Y, Yu A, Yu FX. The Hippo pathway in tissue homeostasis and regeneration. Protein Cell. 2017;8:349–359. doi: 10.1007/s13238-017-0371-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hong AW, Meng Z, Guan KL. The Hippo pathway in intestinal regeneration and disease. Nat. Rev. Gastroenterol. Hepatol. 2016;13:324–337. doi: 10.1038/nrgastro.2016.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Barry ER, et al. Restriction of intestinal stem cell expansion and the regenerative response by YAP. Nature. 2013;493:106–110. doi: 10.1038/nature11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Cai J, et al. The Hippo signaling pathway restricts the oncogenic potential of an intestinal regeneration program. Genes Dev. 2010;24:2383–2388. doi: 10.1101/gad.1978810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Clevers H. The intestinal crypt, a prototype stem cell compartment. Cell. 2013;154:274–284. doi: 10.1016/j.cell.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 197.Gregorieff A, et al. Yap-dependent reprogramming of Lgr5+ stem cells drives intestinal regeneration and cancer. Nature. 2015;526:715–718. doi: 10.1038/nature15382. [DOI] [PubMed] [Google Scholar]
- 198.Azzolin L, et al. YAP/TAZ incorporation in the β-catenin destruction complex orchestrates the Wnt response. Cell. 2014;158:157–170. doi: 10.1016/j.cell.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 199.Swiderska-Syn M, et al. Hedgehog regulates yes-associated protein 1 in regenerating mouse liver. Hepatology. 2016;64:232–244. doi: 10.1002/hep.28542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Oh SH, et al. Liver regeneration requires Yap1-TGFβ-dependent epithelial–mesenchymal transition in hepatocytes. J. Hepatol. 2018;69:359–367. doi: 10.1016/j.jhep.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Fan F, et al. Pharmacological targeting of kinases MST1 and MST2 augments tissue repair and regeneration. Sci. Transl. Med. 2016;8:352ra108. doi: 10.1126/scitranslmed.aaf2304. [DOI] [PubMed] [Google Scholar]
- 202.Porrello ER, et al. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331:1078–1080. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Heallen T, et al. Hippo signaling impedes adult heart regeneration. Development. 2013;140:4683–4690. doi: 10.1242/dev.102798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Lin Z, et al. Cardiac-specific YAP activation improves cardiac function and survival in an experimental murine MI model. Circ. Res. 2014;115:354–363. doi: 10.1161/CIRCRESAHA.115.303632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Xin M, et al. Regulation of insulin-like growth factor signaling by Yap governs cardiomyocyte proliferation and embryonic heart size. Sci. Signal. 2011;4:ra70. doi: 10.1126/scisignal.2002278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Aharonov A, et al. ERBB2 drives YAP activation and EMT-like processes during cardiac regeneration. Nat. Cell Biol. 2020;22:1346–1356. doi: 10.1038/s41556-020-00588-4. [DOI] [PubMed] [Google Scholar]
- 207.Liu Z, et al. MAPK-mediated YAP activation controls mechanical-tension-induced pulmonary alveolar regeneration. Cell Rep. 2016;16:1810–1819. doi: 10.1016/j.celrep.2016.07.020. [DOI] [PubMed] [Google Scholar]
- 208.LaCanna R, et al. Yap/Taz regulate alveolar regeneration and resolution of lung inflammation. J. Clin. Investig. 2019;129:2107–2122. doi: 10.1172/JCI125014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Huang Z, et al. YAP stabilizes SMAD1 and promotes BMP2-induced neocortical astrocytic differentiation. Development. 2016;143:2398–2409. doi: 10.1242/dev.130658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Park R, et al. Yap is required for ependymal integrity and is suppressed in LPA-induced hydrocephalus. Nat. Commun. 2016;7:10329. doi: 10.1038/ncomms10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Poitelon Y, et al. YAP and TAZ control peripheral myelination and the expression of laminin receptors in Schwann cells. Nat. Neurosci. 2016;19:879–887. doi: 10.1038/nn.4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Li J, et al. Matrix stiffening by self-mineralizable guided bone regeneration. Acta Biomater. 2021;125:112–125. doi: 10.1016/j.actbio.2021.02.012. [DOI] [PubMed] [Google Scholar]
- 213.Elbediwy A, et al. Integrin signalling regulates YAP and TAZ to control skin homeostasis. Development. 2016;143:1674–1687. doi: 10.1242/dev.133728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Zhang H, Pasolli HA, Fuchs E. Yes-associated protein (YAP) transcriptional coactivator functions in balancing growth and differentiation in skin. Proc. Natl Acad. Sci. USA. 2011;108:2270–2275. doi: 10.1073/pnas.1019603108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Totaro A, et al. YAP/TAZ link cell mechanics to Notch signalling to control epidermal stem cell fate. Nat. Commun. 2017;8:15206. doi: 10.1038/ncomms15206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Lee MJ, et al. YAP and TAZ regulate skin wound healing. J. Investig. Dermatol. 2014;134:518–525. doi: 10.1038/jid.2013.339. [DOI] [PubMed] [Google Scholar]
- 217.Wang D, et al. Vitamin D3 analogue facilitates epithelial wound healing through promoting epithelial–mesenchymal transition via the Hippo pathway. J. Dermatol. Sci. 2020;100:120–128. doi: 10.1016/j.jdermsci.2020.08.015. [DOI] [PubMed] [Google Scholar]
- 218.Gao Y, et al. Autophagy inhibition facilitates wound closure partially dependent on the YAP/IL-33 signaling in a mouse model of skin wound healing. FASEB J. 2021;35:e21920. doi: 10.1096/fj.202002623RRR. [DOI] [PubMed] [Google Scholar]
- 219.Tapon N, et al. Salvador promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines. Cell. 2002;110:467–478. doi: 10.1016/S0092-8674(02)00824-3. [DOI] [PubMed] [Google Scholar]
- 220.Justice RW, et al. The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev. 1995;9:534–546. doi: 10.1101/gad.9.5.534. [DOI] [PubMed] [Google Scholar]
- 221.Xu T, et al. Identifying tumor suppressors in genetic mosaics: the Drosophila lats gene encodes a putative protein kinase. Development. 1995;121:1053–1063. doi: 10.1242/dev.121.4.1053. [DOI] [PubMed] [Google Scholar]
- 222.Hall CA, et al. Hippo pathway effector Yap is an ovarian cancer oncogene. Cancer Res. 2010;70:8517–8525. doi: 10.1158/0008-5472.CAN-10-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Lu L, et al. Hippo signaling is a potent in vivo growth and tumor suppressor pathway in the mammalian liver. Proc. Natl Acad. Sci. USA. 2010;107:1437–1442. doi: 10.1073/pnas.0911427107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Xia H, et al. LATS1 tumor suppressor regulates G2/M transition and apoptosis. Oncogene. 2002;21:1233–1241. doi: 10.1038/sj.onc.1205174. [DOI] [PubMed] [Google Scholar]
- 225.Zhou D, et al. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell. 2009;16:425–438. doi: 10.1016/j.ccr.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Yu FX, et al. Mutant Gq/11 promote uveal melanoma tumorigenesis by activating YAP. Cancer Cell. 2014;25:822–830. doi: 10.1016/j.ccr.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Hmeljak J, et al. Integrative molecular characterization of malignant pleural mesothelioma. Cancer Discov. 2018;8:1548–1565. doi: 10.1158/2159-8290.CD-18-0804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Eder N, et al. YAP1/TAZ drives ependymoma-like tumour formation in mice. Nat. Commun. 2020;11:2380. doi: 10.1038/s41467-020-16167-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Ammoun S, Hanemann CO. Emerging therapeutic targets in schwannomas and other merlin-deficient tumors. Nat. Rev. Neurol. 2011;7:392–399. doi: 10.1038/nrneurol.2011.82. [DOI] [PubMed] [Google Scholar]
- 230.Siddiqui IA, et al. Resveratrol nanoformulation for cancer prevention and therapy. Ann. N. Y. Acad. Sci. 2015;1348:20–31. doi: 10.1111/nyas.12811. [DOI] [PubMed] [Google Scholar]
- 231.Martinez-Reyes I, Chandel NS. Cancer metabolism: looking forward. Nat. Rev. Cancer. 2021;21:669–680. doi: 10.1038/s41568-021-00378-6. [DOI] [PubMed] [Google Scholar]
- 232.Zhang X, et al. The role of YAP/TAZ activity in cancer metabolic reprogramming. Mol. Cancer. 2018;17:134. doi: 10.1186/s12943-018-0882-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Ayob AZ, Ramasamy TS. Cancer stem cells as key drivers of tumour progression. J. Biomed. Sci. 2018;25:20. doi: 10.1186/s12929-018-0426-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Cordenonsi M, et al. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell. 2011;147:759–772. doi: 10.1016/j.cell.2011.09.048. [DOI] [PubMed] [Google Scholar]
- 235.Cheung P, et al. Regenerative reprogramming of the intestinal stem cell state via Hippo signaling suppresses metastatic colorectal cancer. Cell Stem Cell. 2020;27:590–604. doi: 10.1016/j.stem.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Ma S, et al. Hippo signalling maintains ER expression and ER(+) breast cancer growth. Nature. 2021;591:E1–E10. doi: 10.1038/s41586-020-03131-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Cottini F, et al. Rescue of Hippo coactivator YAP1 triggers DNA damage-induced apoptosis in hematological cancers. Nat. Med. 2014;20:599–606. doi: 10.1038/nm.3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Pearson JD, et al. Binary pan-cancer classes with distinct vulnerabilities defined by pro- or anti-cancer YAP/TEAD activity. Cancer Cell. 2021;39:1115–1134. doi: 10.1016/j.ccell.2021.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Li Z, et al. The Hippo transducer TAZ promotes epithelial to mesenchymal transition and cancer stem cell maintenance in oral cancer. Mol. Oncol. 2015;9:1091–1105. doi: 10.1016/j.molonc.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Minoo P, et al. Prognostic significance of mammalian sterile20-like kinase 1 in colorectal cancer. Mod. Pathol. 2007;20:331–338. doi: 10.1038/modpathol.3800740. [DOI] [PubMed] [Google Scholar]
- 241.Takahashi Y, et al. Down-regulation of LATS1 and LATS2 mRNA expression by promoter hypermethylation and its association with biologically aggressive phenotype in human breast cancers. Clin. Cancer Res. 2005;11:1380–1385. doi: 10.1158/1078-0432.CCR-04-1773. [DOI] [PubMed] [Google Scholar]
- 242.Wang Y, et al. Overexpression of yes-associated protein contributes to progression and poor prognosis of non-small-cell lung cancer. Cancer Sci. 2010;101:1279–1285. doi: 10.1111/j.1349-7006.2010.01511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Zheng X, et al. A novel protein encoded by a circular RNA circPPP1R12A promotes tumor pathogenesis and metastasis of colon cancer via Hippo-YAP signaling. Mol. Cancer. 2019;18:47. doi: 10.1186/s12943-019-1010-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Guo PD, et al. RARgamma downregulation contributes to colorectal tumorigenesis and metastasis by derepressing the Hippo-Yap pathway. Cancer Res. 2016;76:3813–3825. doi: 10.1158/0008-5472.CAN-15-2882. [DOI] [PubMed] [Google Scholar]
- 245.He BP, et al. Differential reactions of microglia to brain metastasis of lung cancer. Mol. Med. 2006;12:161–170. doi: 10.2119/2006-00033.He. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Yates LR, et al. Genomic evolution of breast cancer metastasis and relapse. Cancer Cell. 2017;32:169–184. doi: 10.1016/j.ccell.2017.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Hsu PC, et al. Inhibition of yes-associated protein suppresses brain metastasis of human lung adenocarcinoma in a murine model. J. Cell. Mol. Med. 2018;22:3073–3085. doi: 10.1111/jcmm.13582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Mittal V. Epithelial–mesenchymal transition in tumor metastasis. Annu. Rev. Pathol. 2018;13:395–412. doi: 10.1146/annurev-pathol-020117-043854. [DOI] [PubMed] [Google Scholar]
- 249.Gao Y, et al. YAP inhibits squamous transdifferentiation of Lkb1-deficient lung adenocarcinoma through ZEB2-dependent DNp63 repression. Nat. Commun. 2014;5:4629. doi: 10.1038/ncomms5629. [DOI] [PubMed] [Google Scholar]
- 250.Ma X, et al. Hippo signaling promotes JNK-dependent cell migration. Proc. Natl Acad. Sci. USA. 2017;114:1934–1939. doi: 10.1073/pnas.1621359114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Fan H, et al. ASB13 inhibits breast cancer metastasis through promoting SNAI2 degradation and relieving its transcriptional repression of YAP. Genes Dev. 2020;34:1359–1372. doi: 10.1101/gad.339796.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Cheng Y, et al. LMO3 promotes hepatocellular carcinoma invasion, metastasis and anoikis inhibition by directly interacting with LATS1 and suppressing Hippo signaling. J. Exp. Clin. Cancer Res. 2018;37:228. doi: 10.1186/s13046-018-0903-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Housman G, et al. Drug resistance in cancer: an overview. Cancers (Basel) 2014;6:1769–1792. doi: 10.3390/cancers6031769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168:707–723. doi: 10.1016/j.cell.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Nguyen CDK, Yi C. YAP/TAZ signaling and resistance to cancer therapy. Trends Cancer. 2019;5:283–296. doi: 10.1016/j.trecan.2019.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Zeng R, Dong J. The Hippo signaling pathway in drug resistance in cancer. Cancers (Basel) 2021;13:318. doi: 10.3390/cancers13020318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Wu, Q. et al. YAP drives fate conversion and chemoresistance of small cell lung cancer. Sci. Adv. 7, eabg1850 (2021). [DOI] [PMC free article] [PubMed]
- 258.Qin X, et al. Matrix stiffness modulates ILK-mediated YAP activation to control the drug resistance of breast cancer cells. Biochim Biophys. Acta Mol. Basis Dis. 2020;1866:165625. doi: 10.1016/j.bbadis.2019.165625. [DOI] [PubMed] [Google Scholar]
- 259.Wang X, et al. TFAP2C promotes stemness and chemotherapeutic resistance in colorectal cancer via inactivating hippo signaling pathway. J. Exp. Clin. Cancer Res. 2018;37:27. doi: 10.1186/s13046-018-0683-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Touil Y, et al. Colon cancer cells escape 5FU chemotherapy-induced cell death by entering stemness and quiescence associated with the c-Yes/YAP axis. Clin. Cancer Res. 2014;20:837–846. doi: 10.1158/1078-0432.CCR-13-1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Zhang X, et al. The Hippo pathway transcriptional co-activator, YAP, is an ovarian cancer oncogene. Oncogene. 2011;30:2810–2822. doi: 10.1038/onc.2011.8. [DOI] [PubMed] [Google Scholar]
- 262.Ren A, Yan G, You B, Sun J. Down-regulation of mammalian sterile 20-like kinase 1 by heat shock protein 70 mediates cisplatin resistance in prostate cancer cells. Cancer Res. 2008;68:2266–2274. doi: 10.1158/0008-5472.CAN-07-6248. [DOI] [PubMed] [Google Scholar]
- 263.Yao W, Zhu S, Li P, Zhang S. Large tumor suppressor kinase 2 overexpression attenuates 5-FU-resistance in colorectal cancer via activating the JNK-MIEF1-mitochondrial division pathway. Cancer Cell Int. 2019;19:97. doi: 10.1186/s12935-019-0812-3. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 264.Munoz-Galvan S, et al. Downregulation of MYPT1 increases tumor resistance in ovarian cancer by targeting the Hippo pathway and increasing the stemness. Mol. Cancer. 2020;19:7. doi: 10.1186/s12943-020-1130-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Hao J, et al. Role of Hippo signaling in cancer stem cells. J. Cell. Physiol. 2014;229:266–270. doi: 10.1002/jcp.24455. [DOI] [PubMed] [Google Scholar]
- 266.Panciera T, et al. Induction of expandable tissue-specific stem/progenitor cells through transient expression of YAP/TAZ. Cell Stem Cell. 2016;19:725–737. doi: 10.1016/j.stem.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Bukowski K, Kciuk M, Kontek R. Mechanisms of multidrug resistance in cancer chemotherapy. Int. J. Mol. Sci. 2020;21:3233. doi: 10.3390/ijms21093233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Gujral TS, Kirschner MW. Hippo pathway mediates resistance to cytotoxic drugs. Proc. Natl Acad. Sci. USA. 2017;114:E3729–E3738. doi: 10.1073/pnas.1703096114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Carlino MS, Larkin J, Long GV. Immune checkpoint inhibitors in melanoma. Lancet. 2021;398:1002–1014. doi: 10.1016/S0140-6736(21)01206-X. [DOI] [PubMed] [Google Scholar]
- 270.Robert C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat. Commun. 2020;11:3801. doi: 10.1038/s41467-020-17670-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Wang Z, Wu Z, Liu Y, Han W. New development in CAR-T cell therapy. J. Hematol. Oncol. 2017;10:53. doi: 10.1186/s13045-017-0423-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Bai R, et al. Mechanisms of cancer resistance to immunotherapy. Front. Oncol. 2020;10:1290. doi: 10.3389/fonc.2020.01290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Shi H, et al. Myeloid-derived suppressor cells: implications in the resistance of malignant tumors to T cell-based immunotherapy. Front. Cell Dev. Biol. 2021;9:707198. doi: 10.3389/fcell.2021.707198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Xiao M, et al. Tumor-associated macrophages: critical players in drug resistance of breast cancer. Front. Immunol. 2021;12:799428. doi: 10.3389/fimmu.2021.799428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Wang G, et al. Targeting YAP-dependent MDSC infiltration impairs tumor progression. Cancer Discov. 2016;6:80–95. doi: 10.1158/2159-8290.CD-15-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Guo X, et al. Single tumor-initiating cells evade immune clearance by recruiting type II macrophages. Genes Dev. 2017;31:247–259. doi: 10.1101/gad.294348.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Proietti I, et al. BRAF inhibitors: molecular targeting and immunomodulatory actions. Cancers (Basel) 2020;12:1823. doi: 10.3390/cancers12071823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Subbiah V, Baik C, Kirkwood JM. Clinical development of BRAF plus MEK inhibitor combinations. Trends Cancer. 2020;6:797–810. doi: 10.1016/j.trecan.2020.05.009. [DOI] [PubMed] [Google Scholar]
- 279.Joung J, et al. Genome-scale activation screen identifies a lncRNA locus regulating a gene neighbourhood. Nature. 2017;548:343–346. doi: 10.1038/nature23451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Shalem O, et al. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science. 2014;343:84–87. doi: 10.1126/science.1247005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Kitajima S, et al. Overcoming resistance to dual innate immune and MEK inhibition downstream of KRAS. Cancer Cell. 2018;34:439–452. doi: 10.1016/j.ccell.2018.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Lin L, et al. The Hippo effector YAP promotes resistance to RAF- and MEK-targeted cancer therapies. Nat. Genet. 2015;47:250–256. doi: 10.1038/ng.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Xu W, et al. Up-regulation of the Hippo pathway effector TAZ renders lung adenocarcinoma cells harboring EGFR-T790M mutation resistant to gefitinib. Cell Biosci. 2015;5:7. doi: 10.1186/2045-3701-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Li Z, et al. Loss of the FAT1 tumor suppressor promotes resistance to CDK4/6 inhibitors via the Hippo pathway. Cancer Cell. 2018;34:893–905e898. doi: 10.1016/j.ccell.2018.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Muranen T, et al. ERK and p38 MAPK activities determine sensitivity to PI3K/mTOR inhibition via regulation of MYC and YAP. Cancer Res. 2016;76:7168–7180. doi: 10.1158/0008-5472.CAN-16-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Kim JY, et al. Yap is essential for retinal progenitor cell cycle progression and RPE cell fate acquisition in the developing mouse eye. Dev. Biol. 2016;419:336–347. doi: 10.1016/j.ydbio.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Cabochette P, et al. YAP controls retinal stem cell DNA replication timing and genomic stability. Elife. 2015;4:e08488. doi: 10.7554/eLife.08488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Asaoka Y, et al. The Hippo pathway controls a switch between retinal progenitor cell proliferation and photoreceptor cell differentiation in zebrafish. PLoS ONE. 2014;9:e97365. doi: 10.1371/journal.pone.0097365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Sakabe M, et al. YAP/TAZ-CDC42 signaling regulates vascular tip cell migration. Proc. Natl Acad. Sci. USA. 2017;114:10918–10923. doi: 10.1073/pnas.1704030114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Lee JS, Park HW, Cho KJ, Lyu J. Sestrin2 inhibits YAP activation and negatively regulates corneal epithelial cell proliferation. Exp. Mol. Med. 2020;52:951–962. doi: 10.1038/s12276-020-0446-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Hao GM, et al. The Hippo signaling pathway: a potential therapeutic target is reversed by a Chinese patent drug in rats with diabetic retinopathy. BMC Complement. Alter. Med. 2017;17:187. doi: 10.1186/s12906-017-1678-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Bokhovchuk F, et al. Molecular and structural characterization of a TEAD mutation at the origin of Sveinsson’s chorioretinal atrophy. FEBS J. 2019;286:2381–2398. doi: 10.1111/febs.14817. [DOI] [PubMed] [Google Scholar]
- 293.Matsumoto H, et al. Mammalian STE20-like kinase 2, not kinase 1, mediates photoreceptor cell death during retinal detachment. Cell Death Dis. 2014;5:e1269. doi: 10.1038/cddis.2014.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Lu Q, et al. Heterozygous loss of Yap1 in mice causes progressive cataracts. Investig. Ophthalmol. Vis. Sci. 2020;61:21. doi: 10.1167/iovs.61.12.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Yu W, et al. VGLL4 plays a critical role in heart valve development and homeostasis. PLoS Genet. 2019;15:e1007977. doi: 10.1371/journal.pgen.1007977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Xie J, et al. The role of the Hippo pathway in heart disease. FEBS J. 2021;289:5819–5833. doi: 10.1111/febs.16092. [DOI] [PubMed] [Google Scholar]
- 297.Ciarambino T, Menna G, Sansone G, Giordano M. Cardiomyopathies: an overview. Int. J. Mol. Sci. 2021;22:7722. doi: 10.3390/ijms22147722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Wang P, et al. The alteration of Hippo/YAP signaling in the development of hypertrophic cardiomyopathy. Basic Res. Cardiol. 2014;109:435. doi: 10.1007/s00395-014-0435-8. [DOI] [PubMed] [Google Scholar]
- 299.Chen SN, et al. The hippo pathway is activated and is a causal mechanism for adipogenesis in arrhythmogenic cardiomyopathy. Circ. Res. 2014;114:454–468. doi: 10.1161/CIRCRESAHA.114.302810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Wu W, et al. Activation of Hippo signaling pathway mediates mitochondria dysfunction and dilated cardiomyopathy in mice. Theranostics. 2021;11:8993–9008. doi: 10.7150/thno.62302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Leach JP, et al. Hippo pathway deficiency reverses systolic heart failure after infarction. Nature. 2017;550:260–264. doi: 10.1038/nature24045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Ikeda S, et al. Hippo deficiency leads to cardiac dysfunction accompanied by cardiomyocyte dedifferentiation during pressure overload. Circ. Res. 2019;124:292–305. doi: 10.1161/CIRCRESAHA.118.314048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Wang L, et al. Integrin-YAP/TAZ-JNK cascade mediates atheroprotective effect of unidirectional shear flow. Nature. 2016;540:579–582. doi: 10.1038/nature20602. [DOI] [PubMed] [Google Scholar]
- 304.Mia MM, et al. Loss of Yap/taz in cardiac fibroblasts attenuates adverse remodeling and improves cardiac function. Cardiovasc. Res. 2021;118:1785–1804. doi: 10.1093/cvr/cvab205. [DOI] [PubMed] [Google Scholar]
- 305.Mitani A, et al. Transcriptional coactivator with PDZ-binding motif is essential for normal alveolarization in mice. Am. J. Respir. Crit. Care Med. 2009;180:326–338. doi: 10.1164/rccm.200812-1827OC. [DOI] [PubMed] [Google Scholar]
- 306.Bertero T, et al. Vascular stiffness mechanoactivates YAP/TAZ-dependent glutaminolysis to drive pulmonary hypertension. J. Clin. Investig. 2016;126:3313–3335. doi: 10.1172/JCI86387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Kudryashova TV, et al. HIPPO-integrin-linked kinase cross-talk controls self-sustaining proliferation and survival in pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2016;194:866–877. doi: 10.1164/rccm.201510-2003OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Kudryashova TV, et al. Noncanonical HIPPO/MST signaling via BUB3 and FOXO drives pulmonary vascular cell growth and survival. Circ. Res. 2022;130:760–778. doi: 10.1161/CIRCRESAHA.121.319100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Lv Y, et al. YAP controls endothelial activation and vascular inflammation through TRAF6. Circ. Res. 2018;123:43–56. doi: 10.1161/CIRCRESAHA.118.313143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Chen Y, et al. YAP1/Twist promotes fibroblast activation and lung fibrosis that conferred by miR-15a loss in IPF. Cell Death Differ. 2019;26:1832–1844. doi: 10.1038/s41418-018-0250-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Gokey JJ, et al. Active epithelial Hippo signaling in idiopathic pulmonary fibrosis. JCI Insight. 2018;3:e98738. doi: 10.1172/jci.insight.98738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Eckardt K-U, et al. Evolving importance of kidney disease: from subspecialty to global health burden. Lancet. 2013;382:158–169. doi: 10.1016/S0140-6736(13)60439-0. [DOI] [PubMed] [Google Scholar]
- 313.Drawz P, Rahman M. Chronic kidney disease. Ann. Intern. Med. 2015;162:Itc1–Itc16. doi: 10.7326/AITC201506020. [DOI] [PubMed] [Google Scholar]
- 314.Qian X, et al. YAP mediates the interaction between the Hippo and PI3K/Akt pathways in mesangial cell proliferation in diabetic nephropathy. Acta Diabetol. 2021;58:47–62. doi: 10.1007/s00592-020-01582-w. [DOI] [PubMed] [Google Scholar]
- 315.Chen J, Harris RC. Interaction of the EGF receptor and the Hippo pathway in the diabetic kidney. J. Am. Soc. Nephrol. 2016;27:1689–1700. doi: 10.1681/ASN.2015040415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Cai J, et al. A RhoA-YAP-c-Myc signaling axis promotes the development of polycystic kidney disease. Genes Dev. 2018;32:781–793. doi: 10.1101/gad.315127.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Lee EJ, et al. TAZ/Wnt-beta-catenin/c-MYC axis regulates cystogenesis in polycystic kidney disease. Proc. Natl Acad. Sci. USA. 2020;117:29001–29012. doi: 10.1073/pnas.2009334117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Xu D, et al. Scribble influences cyst formation in autosomal-dominant polycystic kidney disease by regulating Hippo signaling pathway. FASEB J. 2018;32:4394–4407. doi: 10.1096/fj.201701376RR. [DOI] [PubMed] [Google Scholar]
- 319.Xu D, et al. KLF4 initiates sustained YAP activation to promote renal fibrosis in mice after ischemia-reperfusion kidney injury. Acta Pharm. Sin. 2021;42:436–450. doi: 10.1038/s41401-020-0463-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Anorga S, et al. Deregulation of Hippo-TAZ pathway during renal injury confers a fibrotic maladaptive phenotype. FASEB J. 2018;32:2644–2657. doi: 10.1096/fj.201700722R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Xu C, et al. Tubule-specific Mst1/2 deficiency induces CKD via YAP and non-YAP mechanisms. J. Am. Soc. Nephrol. 2020;31:946–961. doi: 10.1681/ASN.2019101052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Hickman RA, O’Shea SA, Mehler MF, Chung WK. Neurogenetic disorders across the lifespan: from aberrant development to degeneration. Nat. Rev. Neurol. 2022;18:117–124. doi: 10.1038/s41582-021-00595-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Gogia N, et al. Hippo signaling: bridging the gap between cancer and neurodegenerative disorders. Neural Regen. Res. 2021;16:643–652. doi: 10.4103/1673-5374.295273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Andreone BJ, Larhammar M, Lewcock JW. Cell death and neurodegeneration. Cold Spring Harb. Perspect. Biol. 2020;12:a036434. doi: 10.1101/cshperspect.a036434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Irwin M, et al. A positive feedback loop of Hippo- and c-Jun-amino-terminal kinase signaling pathways regulates amyloid-beta-mediated neurodegeneration. Front. Cell Dev. Biol. 2020;8:117. doi: 10.3389/fcell.2020.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Gogia N, et al. Inactivation of Hippo and cJun-N-terminal Kinase (JNK) signaling mitigate FUS mediated neurodegeneration in vivo. Neurobiol. Dis. 2020;140:104837. doi: 10.1016/j.nbd.2020.104837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Lee JK, et al. MST1 functions as a key modulator of neurodegeneration in a mouse model of ALS. Proc. Natl Acad. Sci. USA. 2013;110:12066–12071. doi: 10.1073/pnas.1300894110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Mao Y, et al. Targeting TEAD/YAP-transcription-dependent necrosis, TRIAD, ameliorates Huntington’s disease pathology. Hum. Mol. Genet. 2016;25:4749–4770. doi: 10.1093/hmg/ddw303. [DOI] [PubMed] [Google Scholar]
- 329.Yamanishi E, et al. A novel form of necrosis, TRIAD, occurs in human Huntington’s disease. Acta Neuropathol. Commun. 2017;5:19. doi: 10.1186/s40478-017-0420-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Loforese G, et al. Impaired liver regeneration in aged mice can be rescued by silencing Hippo core kinases MST1 and MST2. EMBO Mol. Med. 2017;9:46–60. doi: 10.15252/emmm.201506089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Mooring M, et al. Hepatocyte stress increases expression of yes-associated protein and transcriptional coactivator with PDZ-binding motif in hepatocytes to promote parenchymal inflammation and fibrosis. Hepatology. 2020;71:1813–1830. doi: 10.1002/hep.30928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Bou Saleh M, et al. Loss of hepatocyte identity following aberrant YAP activation: a key mechanism in alcoholic hepatitis. J. Hepatol. 2021;75:912–923. doi: 10.1016/j.jhep.2021.05.041. [DOI] [PubMed] [Google Scholar]
- 333.Wang X, et al. Hepatocyte TAZ/WWTR1 promotes inflammation and fibrosis in nonalcoholic steatohepatitis. Cell Metab. 2016;24:848–862. doi: 10.1016/j.cmet.2016.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Wang X, et al. Cholesterol stabilizes TAZ in hepatocytes to promote experimental non-alcoholic steatohepatitis. Cell Metab. 2020;31:969–986. doi: 10.1016/j.cmet.2020.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Bataller R, Brenner DA. Liver fibrosis. J. Clin. Investig. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Yanguas SC, et al. Experimental models of liver fibrosis. Arch. Toxicol. 2016;90:1025–1048. doi: 10.1007/s00204-015-1543-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Mannaerts I, et al. The Hippo pathway effector YAP controls mouse hepatic stellate cell activation. J. Hepatol. 2015;63:679–688. doi: 10.1016/j.jhep.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 338.Qing J, et al. Dopamine receptor D2 antagonism normalizes profibrotic macrophage-endothelial crosstalk in non-alcoholic steatohepatitis. J. Hepatol. 2022;76:394–406. doi: 10.1016/j.jhep.2021.09.032. [DOI] [PubMed] [Google Scholar]
- 339.Liu Y, et al. Activation of YAP attenuates hepatic damage and fibrosis in liver ischemia-reperfusion injury. J. Hepatol. 2019;71:719–730. doi: 10.1016/j.jhep.2019.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Higashi T, Friedman SL, Hoshida Y. Hepatic stellate cells as key target in liver fibrosis. Adv. Drug Deliv. Rev. 2017;121:27–42. doi: 10.1016/j.addr.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Fujita T, Narumiya S. Roles of hepatic stellate cells in liver inflammation: a new perspective. Inflamm. Regen. 2016;36:1. doi: 10.1186/s41232-016-0005-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Du K, et al. Hedgehog-YAP signaling pathway regulates glutaminolysis to control activation of hepatic stellate cells. Gastroenterology. 2018;154:1465–1479. doi: 10.1053/j.gastro.2017.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Gonzalez-Navajas JM, Lee J, David M, Raz E. Immunomodulatory functions of type I interferons. Nat. Rev. Immunol. 2012;12:125–135. doi: 10.1038/nri3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Chhabra R, Ball C, Chantrey J, Ganapathy K. Differential innate immune responses induced by classical and variant infectious bronchitis viruses in specific pathogen free chicks. Dev. Comp. Immunol. 2018;87:16–23. doi: 10.1016/j.dci.2018.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Wang S, et al. YAP antagonizes innate antiviral immunity and is targeted for lysosomal degradation through IKKvarepsilon-mediated phosphorylation. Nat. Immunol. 2017;18:733–743. doi: 10.1038/ni.3744. [DOI] [PubMed] [Google Scholar]
- 346.He TS, et al. The Hippo signaling component LATS2 enhances innate immunity to inhibit HIV-1 infection through PQBP1–cGAS pathway. Cell Death Differ. 2022;29:192–205. doi: 10.1038/s41418-021-00849-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Zuo, Y. et al. LATS1 is a central signal transmitter for achieving full type-I interferon activity. Sci. Adv. 8, eabj3887 (2022). [DOI] [PMC free article] [PubMed]
- 348.Zhou D, et al. The Nore1B/Mst1 complex restrains antigen receptor-induced proliferation of naive T cells. Proc. Natl Acad. Sci. USA. 2008;105:20321–20326. doi: 10.1073/pnas.0810773105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Mou F, et al. The Mst1 and Mst2 kinases control activation of rho family GTPases and thymic egress of mature thymocytes. J. Exp. Med. 2012;209:741–759. doi: 10.1084/jem.20111692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Abdollahpour H, et al. The phenotype of human STK4 deficiency. Blood. 2012;119:3450–3457. doi: 10.1182/blood-2011-09-378158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Bai X, et al. Mst1 positively regulates B-cell receptor signaling via CD19 transcriptional levels. Blood Adv. 2016;1:219–230. doi: 10.1182/bloodadvances.2016000588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Sakaguchi S, et al. Regulatory T cells and human disease. Annu. Rev. Immunol. 2020;38:541–566. doi: 10.1146/annurev-immunol-042718-041717. [DOI] [PubMed] [Google Scholar]
- 353.Yang J, Sundrud MS, Skepner J, Yamagata T. Targeting Th17 cells in autoimmune diseases. Trends Pharm. Sci. 2014;35:493–500. doi: 10.1016/j.tips.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 354.Shi H, et al. Hippo kinases Mst1 and Mst2 sense and amplify IL-2R-STAT5 signaling in regulatory T cells to establish stable regulatory activity. Immunity. 2018;49:899–914. doi: 10.1016/j.immuni.2018.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Geng J, et al. The transcriptional coactivator TAZ regulates reciprocal differentiation of TH17 cells and Treg cells. Nat. Immunol. 2017;18:800–812. doi: 10.1038/ni.3748. [DOI] [PubMed] [Google Scholar]
- 356.Nehme NT, et al. MST1 mutations in autosomal recessive primary immunodeficiency characterized by defective naive T-cell survival. Blood. 2012;119:3458–3468. doi: 10.1182/blood-2011-09-378364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Johnson R, Halder G. The two faces of Hippo: targeting the Hippo pathway for regenerative medicine and cancer treatment. Nat. Rev. Drug Discov. 2014;13:63–79. doi: 10.1038/nrd4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Barry ER, Simov V, Valtingojer I, Venier O. Recent therapeutic approaches to modulate the Hippo pathway in oncology and regenerative medicine. Cells. 2021;10:2715. doi: 10.3390/cells10102715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Liu Z, et al. Multifunctional nanohybrid based on porous silicon nanoparticles, gold nanoparticles, and acetalated dextran for liver regeneration and acute liver failure theranostics. Adv. Mater. 2018;30:e1703393. doi: 10.1002/adma.201703393. [DOI] [PubMed] [Google Scholar]
- 360.Schirmer AU, et al. Non-canonical role of Hippo tumor suppressor serine/threonine kinase 3 STK3 in prostate cancer. Mol. Ther. 2022;30:485–500. doi: 10.1016/j.ymthe.2021.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Triastuti E, et al. Pharmacological inhibition of Hippo pathway, with the novel kinase inhibitor XMU-MP-1, protects the heart against adverse effects during pressure overload. Br. J. Pharm. 2019;176:3956–3971. doi: 10.1111/bph.14795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Wu Q, et al. Astrocytic YAP protects the optic nerve and retina in an experimental autoimmune encephalomyelitis model through TGF-beta signaling. Theranostics. 2021;11:8480–8499. doi: 10.7150/thno.60031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Bata N, et al. Inhibitors of the Hippo pathway kinases STK3/MST2 and STK4/MST1 have utility for the treatment of acute myeloid leukemia. J. Med. Chem. 2022;65:1352–1369. doi: 10.1021/acs.jmedchem.1c00804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Kastan N, et al. Small-molecule inhibition of Lats kinases may promote Yap-dependent proliferation in postmitotic mammalian tissues. Nat. Commun. 2021;12:3100. doi: 10.1038/s41467-021-23395-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Liu S, et al. Gene therapy knockdown of Hippo signaling induces cardiomyocyte renewal in pigs after myocardial infarction. Sci. Transl. Med. 2021;13:eabd6892. doi: 10.1126/scitranslmed.abd6892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Song S, et al. A novel YAP1 inhibitor targets CSC-enriched radiation-resistant cells and exerts strong antitumor activity in esophageal adenocarcinoma. Mol. Cancer Ther. 2018;17:443–454. doi: 10.1158/1535-7163.MCT-17-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Morice S, et al. The YAP/TEAD axis as a new therapeutic target in osteosarcoma: effect of verteporfin and CA3 on primary tumor growth. Cancers. 2020;12:3847. doi: 10.3390/cancers12123847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Crooke ST, Witztum JL, Bennett CF, Baker BF. RNA-targeted therapeutics. Cell Metab. 2018;27:714–739. doi: 10.1016/j.cmet.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 369.Lihuang L, et al. Targeted combination therapy for glioblastoma by co-delivery of doxorubicin, YAP-siRNA and gold nanorods. J. Mater. Sci. Technol. 2021;63:81–90. doi: 10.1016/j.jmst.2020.03.009. [DOI] [Google Scholar]
- 370.Fitamant J, et al. YAP inhibition restores hepatocyte differentiation in advanced HCC, leading to tumor regression. Cell Rep. 2015;10:1692–1707. doi: 10.1016/j.celrep.2015.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Feng Y, et al. YAP promotes ocular neovascularization by modifying PFKFB3-driven endothelial glycolysis. Angiogenesis. 2021;24:489–504. doi: 10.1007/s10456-020-09760-8. [DOI] [PubMed] [Google Scholar]
- 372.Antisense oligos may hit “undruggable” YAP1. Cancer Discov. 11, OF6–OF6 (2021). [DOI] [PubMed]
- 373.Liu-Chittenden Y, et al. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 2012;26:1300–1305. doi: 10.1101/gad.192856.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Vigneswaran K, et al. YAP/TAZ transcriptional coactivators create therapeutic vulnerability to verteporfin in EGFR-mutant glioblastoma. Clin. Cancer Res. 2021;27:1553–1569. doi: 10.1158/1078-0432.CCR-20-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Dai M, et al. In vivo genome-wide CRISPR screen reveals breast cancer vulnerabilities and synergistic mTOR/Hippo targeted combination therapy. Nat. Commun. 2021;12:3055. doi: 10.1038/s41467-021-23316-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Barrette AM, et al. Anti-invasive efficacy and survival benefit of the YAP-TEAD inhibitor verteporfin in preclinical glioblastoma models. Neuro Oncol. 2022;24:694–707. doi: 10.1093/neuonc/noab244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Ni X, et al. YAP is essential for treg-mediated suppression of antitumor immunity. Cancer Discov. 2018;8:1026–1043. doi: 10.1158/2159-8290.CD-17-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Zhou Y, et al. YAP promotes multi-drug resistance and inhibits autophagy-related cell death in hepatocellular carcinoma via the RAC1–ROS–mTOR pathway. Cancer Cell Int. 2019;19:179. doi: 10.1186/s12935-019-0898-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Chen J, et al. YAP activation in renal proximal tubule cells drives diabetic renal interstitial fibrogenesis. Diabetes. 2020;69:2446–2457. doi: 10.2337/db20-0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Jin J, et al. Inhibition of yes-associated protein by verteporfin ameliorates unilateral ureteral obstruction-induced renal tubulointerstitial inflammation and fibrosis. Int. J. Mol. Sci. 2020;21:8184. doi: 10.3390/ijms21218184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Chen WS, Cao Z, Krishnan C, Panjwani N. Verteporfin without light stimulation inhibits YAP activation in trabecular meshwork cells: Implications for glaucoma treatment. Biochem. Biophys. Res. Commun. 2015;466:221–225. doi: 10.1016/j.bbrc.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 382.Li TY, et al. Critical role of PAFR/YAP1 positive feedback loop in cardiac fibrosis. Acta Pharmacol. Sin. 2022;43:2862–2872. doi: 10.1038/s41401-022-00903-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Bottini A, et al. PTPN14 phosphatase and YAP promote TGFbeta signalling in rheumatoid synoviocytes. Ann. Rheum. Dis. 2019;78:600–609. doi: 10.1136/annrheumdis-2018-213799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Zhang H, et al. Tumor-selective proteotoxicity of verteporfin inhibits colon cancer progression independently of YAP1. Sci. Signal. 2015;8:ra98. doi: 10.1126/scisignal.aac5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Maeda T, Chapman DL, Stewart AF. Mammalian vestigial-like 2, a cofactor of TEF-1 and MEF2 transcription factors that promotes skeletal muscle differentiation. J. Biol. Chem. 2002;277:48889–48898. doi: 10.1074/jbc.M206858200. [DOI] [PubMed] [Google Scholar]
- 386.Pobbati AV, et al. Structural and functional similarity between the Vgll1–TEAD and the YAP–TEAD complexes. Structure. 2012;20:1135–1140. doi: 10.1016/j.str.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 387.Jiao S, et al. VGLL4 targets a TCF4–TEAD4 complex to coregulate Wnt and Hippo signalling in colorectal cancer. Nat. Commun. 2017;8:14058. doi: 10.1038/ncomms14058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Guan X, Fierke CA. Understanding protein palmitoylation: biological significance and enzymology. Sci. China Chem. 2011;54:1888–1897. doi: 10.1007/s11426-011-4428-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Chan P, et al. Autopalmitoylation of TEAD proteins regulates transcriptional output of the Hippo pathway. Nat. Chem. Biol. 2016;12:282–289. doi: 10.1038/nchembio.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Bum-Erdene K, et al. Small-molecule covalent modification of conserved cysteine leads to allosteric inhibition of the TEADYap protein–protein interaction. Cell Chem. Biol. 2019;26:378–389e313. doi: 10.1016/j.chembiol.2018.11.010. [DOI] [PubMed] [Google Scholar]