Table 1.
The potential drugs targeting Hippo pathway in preclinical trials
| Mechanism | Drugs | Structure/sequence | Indications | |
|---|---|---|---|---|
| YAP/TAZ nucleus/cytoplasm location | MSTs kinase activity inhibition | XMU-MP-1 | 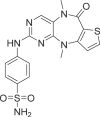 |
Chronic and acute liver injury Protest cancer Breast cancer Autoimmune encephalomyelitis Cardiac hypertrophy |
| SBP-3264 | 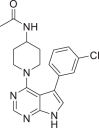 |
Acute myeloid leukaemia | ||
| MSTs kinase expression inhibition | MST1/2-siRNA |
MST1 (5’–3’ sense GAGUGUCAAUAUUGCGAGAtt) MST2 (5’–3’ sense CAAGAGUCAUGAAAAUUGUtt) |
Deficiency of liver regeneration | |
| LATs kinase activity inhibition | TRULI | 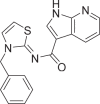 |
No certain indication so far | |
| Sav kinase expression inhibition | Sav-shRNA | Not post |
Myocardial infarction Ischaemic heart failure |
|
| YAP-TEAD transcriptional activity regulation | YAP/TAZ expression inhibition | CA3 | 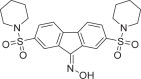 |
Oesophageal adenocarcinoma Osteosarcoma tumour |
| YAP-siRNA | Not post |
Glioblastoma Hepatocellular carcinoma Posterior segment neovascularization-related ocular diseases |
||
| YAP-shRNA | Not post | Lung fibrosis | ||
| YAP-TEAD interaction inhibition | verteporfin | 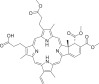 |
Glioblastoma Breast cancer Hepatocellular carcinoma Renal interstitial fibrogenesis Glaucoma |
|
| VGLL4-mimiking peptide | SVDDHFAKSLGDTWLQIGGSGNPK- TANVPQTVPMRLRKLPDSFFKPPE |
Gastric cancer Colorectal cancer |
||
| TEAD palmitoylation inhibition | Flufenamic acid derivative | 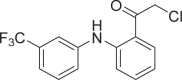 |
Glioblastoma (in vitro) | |
