Fig. 1.
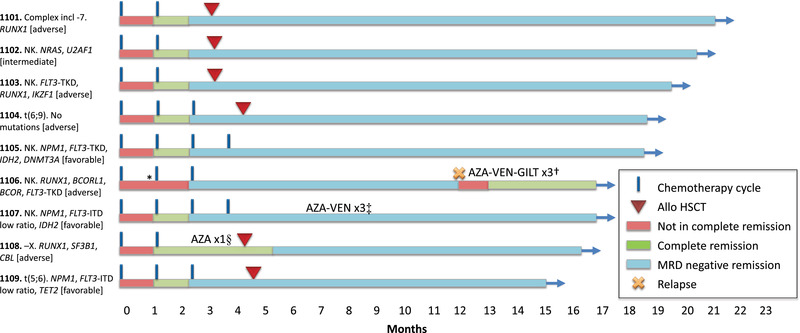
Treatment outcome. The nine patients have been followed for 14.9–20.5 months. Six patients have undergone allogeneic haematopoietic stem cell transplantation (allo‐HSCT). Measurable residual disease (MRD) was performed in accordance with national guidelines after cycle 2 and cycle 4 or prior to allo‐HSCT. In non‐transplanted patients with NPM1, MRD by real‐time quantitative polymerase chain reaction (RT‐qPCR) was performed every 3 months. After allo‐HSCT, patients were routinely monitored every 3 months either by RT‐qPCR (NPM1) or flow cytometry (all others). Eight of nine patients remain in MRD negative remission (as defined by MRD <0.1% by flow cytometry or RT‐qPCR). *, Patient 1106 had bone marrow blasts 5.5% after cycle 1 and did not fulfil complete remission (CR) criteria until after cycle 2. However, blasts may have been elevated by granulocyte colony‐stimulating factor (G‐CSF) usage prior to sampling; †, patient 1106 relapsed after 11.9 months and was treated with a combination of azacitidine–venetoclax–gilteritinib (AZA‐VEN‐GILT) and is now in a second CR; ‡, in patient 1107, NPM1 RT‐qPCR MRD increased from negative to 0.00024%, was rechecked and was then negative again. Before receiving the result of the confirmatory MRD, the patient was put on azacitidine–venetoclax (AZA‐VEN), and three cycles were given as an additional consolidation due to the inconsistent results; §, patient 1108 received one cycle of AZA as bridging while waiting for allo‐HSCT.
