Abstract
An alternative method for the production of renewable fuels from rendered animal fats (pretreated using methods 1–5 or method 7 as described in Annex IV of Commission Regulation (EC) No 2011/142) and used cooking oils, derived from Category 3 animal by‐products, was assessed. The method is based on a catalytic co‐processing hydrotreatment using a middle distillate followed by a stripping step. The materials must be submitted to a pressure of at least 60 bars and a temperature of at least 270°C for at least 4.7 min. The application focuses on the demonstration of the level of reduction of spores from non‐pathogenic spore‐forming indicator bacterial species (Bacillus subtilis and Desulfotomaculum kuznetsovii), based on a non‐systematic review of published data and additional extrapolation analyses. The EFSA BIOHAZ Panel considers that the application and supporting literature contain sufficient evidence that the proposed alternative method can achieve a reduction of at least 5 log10 in the spores of B. subtilis and a 12 log10 reduction in the spores of C. botulinum. The alternative method under evaluation is considered at least equivalent to the processing methods currently approved in the Commission Regulation (EU) No 2011/142.
Keywords: animal fat, Category 3, cooking oil, hydrotreatment, renewable fuel
Summary
On 11 October and on 25 October 2021, the European Food Safety Authority (EFSA) received from the Dutch Competent Authority (Ministry of Agriculture, Nature and Food Quality) the application (EFSA‐Q‐2021‐00625) under Regulation (EC) No 1069/2009 referring to the alternative processing method for animal by‐products (ABP Category 3 material) submitted by BP Raffinaderij Rotterdam B.V. (referred to as bpRR).
The proposed new method has been designed for two specific units (hydrofiners) in the facilities the applicant has in its refinery in Rotterdam (The Netherlands) and involves a catalytic hydrotreatment co‐processing using a middle distillate such as light gasoil (LGO) followed by a stripping step. The materials must be submitted to a pressure of at least 60 bars at a temperature of at least 270°C for at least 4.7 min.
The materials to be treated are rendered animal fats derived from Category 3 materials that have been processed using any of the processing methods 1–5 or processing method 7 (as described in Annex IV of Com Reg (EU) No 142/2011) and used cooking oil (UCO) not treated with any processing method. The BIOHAZ Panel clarified that UCO is considered catering waste and catering waste could be Category 1 or Category 3 animal by‐products (ABP), as per Article 10 (p) of Regulation (EC) No 1069/2009. Only Category 3 UCO must be used to produce renewable fuels with the proposed method.
The EFSA BIOHAZ Panel considered that a reduction of 5 log10 and 3 log10 of the relevant pathogenic bacteria and thermoresistant viruses, respectively, as defined in the hazard identification, should be demonstrated to validate the alternative method. If spore‐forming pathogenic bacteria are considered relevant in the hazard identification, the required level of inactivation should be a 5 log10 reduction of spores from pathogenic bacteria, with the exception of spores of C. botulinum, for which a 12 log10 reduction will be required, as for processing canned petfood. If needed/appropriate, for both spore‐forming and non‐spore‐forming bacteria and viruses, adequately justified alternative non‐pathogenic indicator organisms with at least the same level of resistance may be used, demonstrating at least a similar level of reduction of all biological hazards possibly present in the Category 3 material.
Given the possibility of the presence of various pathogens including spore‐forming bacteria, depending on source and location, the applicant used, based on a literature search and due to their high thermal resistance, spores of Bacillus subtilis and Desulfotomaculum kuznetsovii as indicator organisms to demonstrate the level of hazard reduction. Despite not conducting a full hazard identification process, the approach followed by the applicant is consistent with one of the possible scenarios considered acceptable: when no full hazard identification is conducted, the selection of spores from non‐pathogenic spore‐forming indicator bacterial species as a primary target to demonstrate a sufficient level of hazard reduction, considering that any process achieving a significant level of inactivation of them will ensure at least a similar level of reduction of all biological hazards possibly present in the Category 3 material.
The applicant presented a body of evidence for the level of hazard reduction based on a non‐systematic literature review and the estimation of the log10 reduction at the minimum temperature proposed by the alternative method (270°C) through extrapolating from available data at lower heating temperatures in publicly available studies. However, data extrapolated beyond the interpolation region was not considered in the assessment since the extrapolation analyses performed by the applicant have limitations. Despite these considerations, the dossier and additional literature contain sufficient evidence to support that the proposed alternative method can achieve a sufficient level of hazard reduction (e.g. a reduction of at least 5 log10 in the spores of B. subtilis and a 12 log10 reduction in the spores of C. botulinum).
In the Hazard Analysis and Critical Control Point (HACCP) plan, the reactors and product strippers were identified by the applicant as CCPs and this was considered to be correct. The critical limits, means of monitoring and verification, and corrective actions associated to the CCPs were clear, except for the means of verification of the successful entry and exit of the materials in the stripper tower. The applicant identified the acceptance of the material on site as a CCP while this should be a prerequisite. The information provided by the applicant indicates that comprehensive and adequate procedures are in place for dealing with any risks associated with interdependent processes and the end use of the product. Overall, the alternative method under assessment is considered at least equivalent to the processing methods currently approved in the legislation.
1. Introduction
1.1. Background
On 11 October and 25 October 2021, the European Food Safety Authority (EFSA) received from the Dutch Competent Authority (Ministry of Agriculture, Nature and Food Quality) the application (EFSA‐Q‐2021‐00625) under Regulation (EC) No 1069/2009 1 referring to the alternative processing method for animal by‐products (ABP Category 3 material) submitted by BP Raffinaderij Rotterdam B.V. (referred to as bpRR).
The applicant submitted an application as required in the procedure for authorisation of an alternative method of use or disposal of animal by‐products (ABP) or derived products, laid down in Article 20 of the Regulation (EC) No 1069/2009.
During the completeness check, performed according to Regulation (EC) No 1069/2009, it was noticed that some information was missing or incomplete. On 8 December 2021, EFSA sent a letter to the applicant with the following five requests, which referred to the sections of the dossier as provided:
In Section 4.1.1 of Annex 1, the applicant listed the microbiological hazards that could remain in the rendered fats derived from Category 3 materials (AF), ‘that may include Salmonella, Enterobacteriaceae and spore‐forming bacteria such as Clostridium perfringens’. We ask the applicant to please clarify the selection criteria of these microbiological hazards, keeping into consideration the provisions of the EFSA guidelines (p. 6 – https://www.efsa.europa.eu/en/efsajournal/pub/1680) that indicate that the relevant biological hazards for human and animal health should be related to the category and subcategory of the material to be processed and that the biological agent/s that are the most difficult to be inactivated by the critical parameters defined in the full description of the process (e.g. thermoresistant micro‐organisms) should be retained as the primary target/s for demonstrating the risk reduction achieved by the process.
According to the description in Section 2.6, two hydrofiners (GOH1 and GOH3) will be used to process the mixture of hydrocarbons and animal fats (AF) and used cooking oils (UCO). The specifications of these units result in different operating conditions. Therefore, there are two described processes with similar steps and reactions but with different combinations of time/temperature/pressure, which are critical parameters for the evaluation of the level of risk reduction. As described in the EFSA guidelines (link above): ‘The parameters that are critical for the inactivation of the pathogens (e.g. temperature, pressure, exposure time, pH, particle size) shall be stated in relation to the process’. Even though, in Section 5.2, the applicant applied the worst case scenario of the conditions in the two hydrofiners: ‘in the environments described in both hydrofiners the temperature exceeds 270°C and exhibit pressures of at least 60 barg for at least 4.7 min’, we ask the applicant to include in the dossier (in the section ‘Full description of the process’) a univocal generic description of the physical/chemical steps of the process with the parameters that are critical for the inactivation of the pathogens, irrespective of its implementation in the two hydrofiners.
The applicant provided in Sections 4 and 6 some information on the risk associated with interdependent processes and the risk associated with intended end use of the products. However, these two points should be covered separately in stand‐alone sections of the application, as indicated in the above‐mentioned EFSA guidelines (page 8) and in Regulation 142/2011 2 , Annex VII, Chapter II: Content of applications. Therefore, we ask the applicant to please update the application accordingly.
During its review, EFSA has identified no specific claims for confidentiality (Annex 1 is marked with a generic ‘Draft – Confidential’ in each page). If applicable and in accordance with Art. 39 of Regulation 178/2002 3 , we ask the applicant to please clearly identify the specific aspects of the application for which confidentiality treatment is requested by specifying the applicable excerpt(s) or data sets, and figure(s) or diagram(s) in the dossier as well as a verifiable justification(s)/reasons(s) for the confidentiality requests. The confidential parts should be clearly boxed or earmarked or highlighted in the application. Alternatively, the applicant is asked to confirm that no claims for confidentiality are made for this application.
The applicant has indicated ‘Draft’ at the bottom of each page of Annex 1. If Annex 1 is considered as the final application, the wording ‘draft’ should be removed. We ask the applicant to please clarify the meaning of this wording or to remove it from the application.
On 26 January 2022, EFSA received a new version of the dossier in which the points above had been addressed. The list of documents submitted to EFSA is available in Section 5. After checking the content of the full dossier, EFSA considered that the application EFSA‐Q‐2021‐00625 was valid on 9 February 2022. According to Regulation (EC) No 1069/2009, EFSA shall respect the deadline of 6 months to deliver the scientific opinion. Therefore, the opinion must be delivered by 9 August 2022.
1.2. Additional information
During the discussions of the content of the dossier, the Working Group (WG) agreed on two separate occasions that there was a need to request to the applicant further clarifications and additional information on specific points. The first request was sent on 3 March 2022, to which the applicant submitted on 18 March 2022 a modified version of the application addressing the questions accordingly and for purposes of clarity, as requested, including information about pretreatments and production of by‐products, the HACCP plan, the co‐processing using light gas oil (LGO) and the preprocessing of UCOs.
After a further review on 18 May 2022, the WG decided to stop the clock until the receipt of an amended dossier with further clarifications on the hazard identification, demonstration of the level of risk reduction, and details of the HACCP plan and of the risk of interdependent processes. The applicant submitted an amended Annex I (Application for alternative method for the processing of ABP at BP Rotterdam Refinery) and three new Appendices (7, 8, 9) on 12 July 2022. The clock restarted again on the 18 July 2022, when EFSA confirmed the acceptance of the amended dossier by letter. The new deadline for delivery of the opinion was set at the 10 October 2022.
2. Data and methodologies
2.1. Data
The data used in the assessment were provided by the applicant as requested in Annex VII of Commission Regulation (EU) No 142/2011 and its amendment by Regulation (EU) No 749/2011 4 . A process flow diagram (Figure 1 and Appendix A) and a Hazard Analysis and Critical Control Points (HACCP) plan were included in the application dossier. The report submitted by the Dutch Competent Authority (CA) related to the application was also considered. Relevant scientific papers provided by experts of the WG and previous EFSA opinions were also considered during the assessment.
Figure 1.
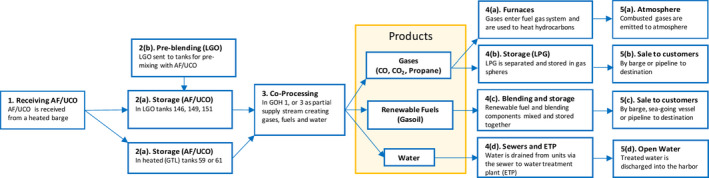
Flow diagram of the process to produce renewable fuels from AF and UCO
2.2. Methodologies
The EFSA BIOHAZ Panel evaluated the application for an alternative processing method for Category 3 ABP by individually assessing the following steps as set out in the ‘Statement on technical assistance on the format for applications for new alternative methods for animal by‐products’ (EFSA BIOHAZ Panel, 2010). These steps are:
a full description of the process;
a full description of the material to be treated;
hazard identification;
the level of risk reduction 5 ;
the HACCP plan;
the risk associated with interdependent processes;
the risk associated with the intended end use of the products.
The applicant is required to document as fully as possible the different aspects of each of these steps. According to the CA assessment, the application meets the requirements as laid down in the EFSA Statement (EFSA BIOHAZ Panel, 2010).
As set out in subparagraph 5 of Article 20 of Regulation (EC) No 1069/2009, EFSA shall assess whether the method submitted ensures that the risks to public or animal health are: ‘controlled in a manner that prevents their proliferation before disposal in accordance with this Regulation or their implementing measures; or reduced to a degree that is at least equivalent, for the relevant category of ABP, to the processing methods laid down pursuant to point (b) of the first subparagraph of Article 15(1)’.
2.2.1. Review of the standards applied in previous EFSA opinions
According to point 2d, Chapter II, Annex VII of Commission Regulation (EU) No 142/2011, any application for the evaluation of alternative methods shall ‘show that the most resistant biological hazards associated with the category of materials to be processed are reduced in any products generated during the process, including the wastewater, at least to the degree achieved by the processing standards laid down in this Regulation for the same category of animal by‐products. The degree of risk reduction must be determined with validated direct measurements, unless modelling or comparisons with other processes are acceptable’.
According to the EFSA Statement (EFSA BIOHAZ Panel, 2010) and to point 3, Chapter II, Annex VII of Commission Regulation (EU) No 142/2011, validated direct measurements as referred to above shall mean:
- ‘measuring the reduction of viability/infectivity of endogenous indicator organisms during the process, where the indicator is:
- consistently present in the raw material in high numbers,
- not less resistant to the lethal aspects of the treatment process, but also not significantly more resistant, than the pathogens for which it is being used to monitor,
- relatively easy to quantify and relatively easy to identify and to confirm; or
using a well‐characterised test organism or virus introduced in a suitable test body into the starting material’.
The EFSA Statement (EFSA BIOHAZ Panel, 2010) also asserts that ‘results should be accompanied by evidence’. Such evidence ‘includes, for measurements, information on the methodology used, nature of samples that have been analysed and evidence that samples are representative (e.g. number of samples, number of tests performed and selection of measuring points). If several treatment steps are involved, an assessment should be performed on the degree to which individual titre reduction steps are additive, or whether early steps in the process may compromise the efficacy of subsequent steps. In any case it is necessary to provide the sensitivity and specificity of the detection methods applied. Data on the repeatability and statistical variability of the measures obtained during the experiments should also be presented’. It also states that ‘generally, the level of risk reduction for human and animal health which can be achieved by the process should be evaluated on the basis of direct measurements (validation)’.
‘In case no direct measurements of the risk reduction be available (i.e. no validation as defined above is feasible), modelling or comparison with other processes may be acceptable if: the factors leading to the risk reduction are well known; the model of risk reduction is well established; and continuous direct measurements of the factors leading to the risk reduction are provided for the full‐scale process which demonstrate that these factors are homogeneously applied throughout the treated batch’.
In point 2d, ‘Level of risk reduction’ of Section 2.1.2.1 ‘Content of applications’ of the EFSA Statement (EFSA BIOHAZ Panel, 2010), it is stated that ‘in principle, the new proposed process should be able to reduce the amount of the most resistant biological hazards associated with the category of the material to be processed for a defined final use to an acceptable level’. Although Chapter II of Annex VII of Commission Regulation (EU) No 142/2011 adopted the proposal of the EFSA Statement to use ‘the level of risk reduction’ and ‘the level of reduction of the most resistant biological hazards’ interchangeably, it is acknowledged that these are different terms and that the purpose of the evaluation of alternative methods is not the estimation of the level of any risk, but the level of hazard reduction. It is acknowledged that the level of reduction described above may result in different levels of safety for humans and animals according to the ultimate end use of the product: renewable fuels, biogas, composted material, organic fertiliser, or any other.
The standard processing methods for the different categories of ABP are described in Chapter III, Annex IV of Commission Regulation (EU) No 142/2011. There are no hazard reduction standards for proposed alternative methods for biodiesel or renewable fuels production using ABP. However, in previous EFSA opinions (EFSA BIOHAZ Panel, 2015a, 2017, 2020a) dealing with proposed alternative processing methods including Category 1 ABP, the BIOHAZ Panel concluded that a reduction of 6 log10 in prion infectivity by the alternative method is required to consider it at least equivalent to the method approved in the legislation, disregarding the level of inactivation achieved by the pretreatment (method 1). When the starting material is Category 3, the degree of hazard reduction (or level of risk reduction) achieved by the approved methods is not specified, and no definitive standards have been set down either in relation to hazard reduction for alternative methods dealing with Category 3 materials. This was already highlighted by previous EFSA opinions. For example, in the EFSA Statement on technical assistance related to the EFSA Opinion on transformation of ABPs into biogas and compost (EFSA BIOHAZ Panel, 2009), the Panel recommended that ‘requirements for the reduction of the representative pathogens or indicators should be defined according to the final use of the different ABP categories to be processed, with the different ABP categories representing different risks of microbiological contamination of the input material’.
There have been precedents of assessments conducted by EFSA on alternative methods for Category 3 ABP in combination with other categories or alone, but not for the production of biodiesel or renewable fuels, in which different levels of risk reduction were required, depending on the end use and the legal requirements.
For feed, the EFSA Scientific Opinion on an alternative method for the hygienic treatment of bovine colostrum through a series of filtration steps (EFSA BIOHAZ Panel, 2015b) compared the level of inactivation with the one achieved by the standard required, which in that case was high‐temperature short time (HSTS) pasteurisation at 72°C for at least 15 s or an equivalent pasteurisation effect achieving a negative reaction to a phosphatase test. More recently, in the EFSA Opinion on evaluation of the application for a new alternative processing method for ABP of Category 3 material (ChainCraft B.V.) for feed use (EFSA BIOHAZ Panel, 2018), the efficacy of the process was evaluated based on the ability of that physical process to remove potential biological hazards present in the material. The standard applied followed the level of agent risk reduction applied in the 2005 EFSA Opinion on the safety vis‐à‐vis biological risks of biogas and compost treatment standards of animal by‐products (ABP) (EFSA BIOHAZ Panel, 2005) (see below). The EFSA Scientific Opinion on hatchery waste as animal by‐products (EFSA BIOHAZ Panel and EFSA AHAW Panel, 2011) is also relevant in this discussion even though hatchery waste is officially designated as Category 2. This opinion stated that the risk related to the use of dead‐in‐shell chicks for the production of processed petfood submitted to a conventional heat treatment to a minimum of 121°C for 3 min in a moist environment, was considered negligible. However, the level of inactivation of the identified biological hazards achieved by any of the methods assessed was not specified and it was concluded that ‘a treatment of at least 90°C throughout the substance of the final product is not able to inactivate other relevant hazards such as bacterial spores, thermoresistant viruses and some toxins. The final risk posed by the agents that may survive this treatment additionally depends on several factors and cannot be considered to be negligible’.
For organic fertilisers and soil improvers, the opinion of the Scientific Panel on Biological Hazards of the European Food Safety Authority on the safety vis‐à‐vis biological risk, including for transmissible spongiform encephalopathies (TSEs), of the application on pastureland of organic fertilisers and soil improvers (EFSA BIOHAZ Panel, 2004) stated that ‘despite the fact that the ABP classed as Category 3 come from animals considered fit for human consumption, there is no absolute guarantee that TSE infective material would not be present in the material (e.g. animals in early stage of incubation not picked up by rapid testing)’. It was recommended that ‘the validation studies should be carried out using test organisms that have shown to be a good model for microbiological hazards potentially present in the process and/or product. The test organisms used should at least be as resistant as micro‐organisms potentially present. The test organisms should be applied under the same conditions as how they appear to be in the raw material. The decontamination must achieve a 5 log10 reduction’.
For biogas and compost, the 2005 EFSA Opinion on the safety vis‐à‐vis biological risks of biogas and compost treatment standards of ABPs (EFSA BIOHAZ Panel, 2005) considered the process under evaluation equivalent to the processing standards laid down in Regulation 6 , ‘if the treatment was capable of reducing the concentration of the relevant pathogenic bacteria by at least 5 log10 and the infectious titre of the relevant viruses by at least 3 log10’. This opinion recommended that any process for hazard reduction should be validated with representative agents in relation to the reduction target defined and must prove that ‘the process achieves the following (for thermal processes, condition (a) and (b) have to be fulfilled; for chemical processes condition (c) also has to be fulfilled):
Reduction of 5 log10 of non‐spore forming pathogenic bacteria, of parasites and of non‐thermoresistant viruses. Reduction of Enterococcus faecalis indicates an equivalent or even higher reduction of viable non‐spore forming bacteria (e.g. Salmonella, Enterobacteriaceae), of parasites and of infectious non‐thermoresistant viruses (e.g. foot and mouth disease virus, classical swine fever virus). In theory, the treatment required by the current legislation (70°C, 60 min) achieves this reduction.
Reduction of infectivity titre of thermoresistant viruses by a minimum of 3 log10, whenever they are identified as a relevant hazard. According to the little scientific information available, the treatment required by the current legislation does not achieve this reduction.
Reduction of parasites by at least 99.9% (3 log10) of viable stages’.
This standard was applied in the EFSA Opinion on the safety vis‐à‐vis biological risk of the mesophilic process of biogas and compost treatment of ABPs (EFSA BIOHAZ Panel, 2007): ‘to reduce the number of the relevant pathogenic bacteria by at least 5 log10, the infectious titre of the relevant thermoresistant viruses by at least 3 log10, whenever they are a relevant hazard, and the number of viable parasitic stages by at least 3 log10 in the given exposure time’. The EFSA Scientific Opinion on the risk to public and/or animal health of the treatment of dead‐in‐shell chicks (Category 2 material) to be used as raw material for the production of biogas or compost with Category 3 approved method (EFSA BIOHAZ Panel, 2015c) applied also the alternative biogas and composting standards for Category 3 material of 5 log10 for vegetative bacteria and 3 log10 for viruses.
The 2010 EFSA Statement on the technical assistance on the format for applications for new alternative methods for animal by‐products (EFSA BIOHAZ Panel, 2010) states that the ‘standard already approved for validation of composting processes for Category 3 ABPs can be used as a benchmark for other treatment processes for comparable input material and potential end use’.
The standards applied in these early opinions were considered by the regulator in the requirements for the approval of alternative transformation parameters for biogas and composting plants in terms of the validation of the intended process, referred to in point 1, Section 2, Chapter III, Annex V of Commission Regulation (EU) No 142/2011.
2.2.2. Standards to be applied for Category 3 material
In order to be considered at least equivalent to the processing methods approved in the legislation, the alternative methods for Category 3 ABP should be capable of reducing the concentration of the relevant pathogenic bacteria by at least 5 log10 and the infectious titre of the relevant viruses by at least 3 log10 (EFSA BIOHAZ Panel, 2005). For chemical treatments, a reduction of viable stages of resistant parasites such as eggs of Ascaris sp. by at least 99.9% (3 log10) shall be required. The determination of the relevant pathogenic bacteria and viruses should be defined by the hazard identification, specific for the material to be treated.
If the hazard identification considers spore‐forming pathogenic bacteria to be relevant, the required level of inactivation will also be a 5 log10 reduction of spores from these bacteria, with the exception of spores of C. botulinum for which a 12 log10 reduction would be required, as for processing canned petfood. This is the expected reduction in C. botulinum spores after applying 121.1°C for 3 min, the minimum standard of a heat treatment for canned petfood. 7
Given their well‐described high level of resistance to thermal and chemical treatments, applicants may choose to directly use spores of pathogenic bacteria as primary indicators without carrying out a full hazard identification exercise.
If needed/appropriate, for both spore‐forming and non‐spore‐forming bacteria and viruses, adequately justified alternative non‐pathogenic indicator or surrogate organisms with at least the same level of resistance may be used, demonstrating an equivalent level of reduction in the substrate of interest.
These reductions should be achieved by the process independently from the reduction provided by the standard processing methods [methods 1–5 or 7 of Commission Regulation (EU) 2011/141], should these be required.
3. Assessment
In the current chapter, the sections defined as ‘provided by the applicant’ present the description extracted verbatim from the application, edited for clarity and abridged in places for brevity.
3.1. Description of the alternative method
3.1.1. Description of the alternative method as provided by the applicant
The alternative method has been designed for two specific units (hydrofiners) in the facilities the applicant has in its refinery in Rotterdam (The Netherlands). The AF and UCO (or AF and UCO mixture with LGO) will be sent to the feed system of one of two hydrofiners. The feed system will combine various streams of hydrocarbons including those not only from the AF and UCO but also from other storage tanks as well as other process installation units including the crude distillation units (CDUs). All other streams that are mixed with the AF and UCO will have already undergone some form of processing and distillation.
Two hydrofiners (GOH1 and GOH3) will be used to process the mixture of hydrocarbons and AF and UCO. The hydrofiners will simultaneously process all material (co‐process). The co‐processing of AF and UCO will take place in these units consisting of hydrogenation and decarboxylation of fatty acids to produce distillate, on top of the standard hydrotreatment of the gas oil fed to the unit.
The hydrofiners are facilities within the refinery that induce chemical reactions and transform AF and UCO into the final product. They are located in the crude distillation area (CDU) section of the plant. The primary purpose of the hydrofiners is hydrotreatment. Hydrotreatment occurs within a high‐pressure and temperature environment where hydrogen is heavily consumed. The main reactions that occur cause the removal of sulfur (desulfurisation) and nitrogen (denitrification) that enables saturation of hydrocarbon molecules. During this process, metals and oxygen compounds are removed as well.
The following chemical reactions will take place in the reactors:
Desulfurisation: it is the most important reaction, and it removes the sulfur that is joined to the hydrocarbon by a chemical bond. The sulfur is then converted to hydrogen sulfide (H2S). This reaction currently occurs with fossil fuel hydrocarbons. When AF and UCO are introduced that contain sulfur, the reaction will occur in parallel.
Denitrification: it removes nitrogen that is chemically bonded to the hydrocarbon chain. The nitrogen is converted to ammonia (NH3). This reaction currently occurs with fossil fuel hydrocarbons. When AF and UCO are introduced that contain nitrogen, the reaction will occur in parallel.
Olefin saturation: double bond saturation in the hydrocarbon chains. It occurs very rapidly and with much heat release. This reaction occurs with fossil fuel hydrocarbons and will also occur when AF and UCO are introduced.
Aromatics saturation: these are the most difficult reactions, and they consist of saturating the double bonds in the cyclic hydrocarbon molecules. They are less exothermic than the reactions above.
Hydrogenation and decarboxylation: these reactions will occur in AF and UCO. If the carbon chain has a double or triple bond, they will be saturated (hydrogenation). These molecules are not aromatic but are all long chained hydrocarbons. Decarboxylation forms three n‐paraffins, methane, propane and water. In the hydrodeoxygenation reaction, the hydrogen saturates the unsaturated bonds and extracts the oxygen from the triglyceryls that are present in the fats and oils, obtaining gas oil (CnHn+2), which is a pure paraffinic product and a co‐product of propane (C3H8), with by‐products being CO2, CO and water. The reaction is shown below:
The final step is the stripping. The purpose is to further separate H2S, CO, CO2, propane and light hydrocarbon fractions (gases) from the gas oil stream. The principles are based on steam distillation and operate as a function of the temperature and pressure of the product stripper. Steam is injected at the bottom of the column to achieve the required flash point specifications of the product and flows to the top of the tower. The lightest gases (including CO, CO2 and propane) exit the top of the tower and once again are separated, scrubbed and sent to the fuel gas system. Part of the gases (mainly propane) constitute wild naphtha and are sent to the other hydrotreaters (DHTs) for further processing. These gases eventually are separately stored in spheres and sold.
A part of the light hydrocarbon fractions is returned to the stripper to control the temperature at the top of the tower. The tower has a temperature profile whereby the top of the tower is approximately 170°C and the bottom of the tower is 240°C. The liquid product is produced on a level control as ‘stabilised naphtha’.
Gas oil travels through numerous trays to before reaching the bottom of the tower. During this process, the gas oil becomes a high‐quality distillate (kerosene, diesel and fuel oils) that is produced at the bottom of the stripper. This stream is wet and therefore dried in a vacuum drier before it is sent to the air coolers and from there to the automotive diesel oil (ADO) blender. The retention time of the liquid in the stripping tower could not be calculated due to the design of the tower including its trays.
As direct stripping steam injection is being used, acid water is produced in the overhead drum. The acid water is used as wash‐water and injected upstream of the air coolers. During start‐up, fresh water is used to establish the wash‐water circuit. The excess acid water is mixed with other streams and sent to treatment.
There are other types of reactions that also take place in the reactors on a lower scale (metals trapping, oxygen compounds elimination, hydrocracking reactions). However, these reactions do not significantly contribute to the hydrogen consumption or heat release but can have an important effect on the catalyst deactivation rate.
Once the reactions are complete, the renewable fuel (gas oil) will be sent to the ADO blender for final inline blending. Product specifications may vary depending on the destination of the country and its respective requirements. Samples are taken of the final product to ensure that it meets proper specifications (this has also been identified as a critical control point within the process that is discussed later). Specifications are derived from international standards on the production of renewable fuels.
Due to the flexibility and range of products bpRR will create the final renewable fuel (gas oil – end product) whereby AF and UCO reside may vary. However, for the final product to be placed on the market, the final product must comply with standard specifications (e.g. ISO 8217, EN 228, EN 590 or Defstan 9191).
The gases, including propane, CO and CO2, are partially recycled and sent to the existing fuel gas system of the refinery. The fuel gas system is a network that imports natural gas from a provider and also uses fuel gas (a mixture of various gases that are distilled during the refining process) and provides fuel to the furnaces on site. The remaining part of the gases are part of the wild naphtha products and are sent for further processing and will form part of the liquid propane gases (LPG).
The by‐products created during this process are handled separately. Propane is partially consumed by on‐site furnaces to preheat hydrocarbons. Part of the propane that is produced is sent to the LPG spheres and exported from the site as a final product. Gases such as CO and CO2 will separate and, mixed with the existing fuel gas system, will be emitted to the atmosphere. Emissions to the atmosphere are regulated through bpRR's environmental permit granted through the General Environmental Provisions Act [Wet algemene bepalingen omgevingsrecht [General Provisions Environmental Law Act] (Wabo)] by the local environmental authorities (DCMR).
Water is separated and drained into the designated oily‐water sewer system. The sewer is connected to the water effluent treatment plant (ETP) that processes all the water from the refinery. It is a biological treatment plant that has been designed in accordance with best available techniques (BAT), with technologies that have been identified and selected as industry standards. Once treated, the water is discharged into the sea. bpRR has received a permit from the executive agency of the Ministry of Infrastructure and Water Management (Rijkswaterstaat) for discharging water into the local harbour.
The operating conditions and retention time of equipment of the gas oil hydrofiners GOH3 and GOH1 are shown in Table 1, as included in the application, and the process flow diagrams are displayed in Appendix A.
Table 1.
Operating conditions and retention time of equipment of the gas oil hydrofiners GOH3 (above) and GOH1 (below)
| Equipment | Pressure (barg) | Temperature (°C) | Minimum retention time (min) |
|---|---|---|---|
| GOH3 | |||
| D2301 – Feeder | 3 | 120 | 6.5 |
| H2301 – Furnace | 65 | 290–350 | 0.2 |
| R2301 – Reactor | 65 | 350–400 | 6 |
| R2302 – Reactor | 65 | 350–400 | 6 (in parallel with R2301) |
| D2302 – Separator | 64 | 240–250 | 3 |
| D2303 – Separator | 64 | 40 | 4.8 |
| T2301 – Stripper | 5.5 | 170–240 | – |
| GOH3 total | > 23 (a) | ||
| GOH1 | |||
| D806 – Feeder | 1 | 88 | 14.7 |
| H801 – Furnace | 60 | 270–320 | 1 |
| R801 – Reactor | 60 | 320–405 | 1 |
| R802 – Reactor | 60 | 320–405 | 2.7 |
| D801 – Separator | 60 | 40 | 6.5 |
| D802 – Separator | 60–67 | 40 | 5.2 |
| T801 – Stripper | 7 | 170–300 | – |
| GOH1 total | – | – | > 36.2 (a) |
Time does not include material travelling through stripping tower T2301.
Generic process description
The following section provides an overall general description that should encompass both hydrofiners.
Multi‐step catalytic hydrotreatment co‐processing for the production of renewable fuels
1. Starting materials
For this process, the following materials may be used:
Rendered fats derived from Category 3 material, which have been processed using any of the processing methods 1–5 or processing method 7, or UCO (catering waste) defined as Annex 1 point 22 of Regulation 142/2011;
A middle distillate deemed as a suitable feedstock for a hydrotreatment process;
The use of rendered fats derived from Category 1 or Category 2 material for this process shall be prohibited.
2. Processing method
The starting materials (namely rendered fats and middle distillate) shall be processed simultaneously through a hydrotreatment process.
The materials must be submitted to a hydrotreatment process that consists of a catalytic hydrotreatment step followed by a stripping step.
The materials must be submitted to a pressure of at least 60 bars at a temperature of at least 270°C for at least 4.7 min.
3.2. Material to be treated
3.2.1. Material to be treated as provided by the applicant
All AF and UCO provided to the refinery must be in liquid form free from solids. It shall not contain recycled oils (lubricants), waste oils (hydraulic fluids, sewage sludge), mineral oils (fossil products) or fish oils as this is part of the contaminant control.
Animal fats are rendered fats derived from Category 3 material that includes material that was previously found ‘fit for human consumption’, including raw meat, hides and skins; parts of slaughtered animals that are fit for human consumption but that are not intended for human consumption for commercial reasons, or due to problems of manufacturing or packaging defects or by‐products derived from the processing of products intended for human consumption (e.g. degreased bones and greaves) and blood from healthy ruminants.
AF that have been processed using any of the processing methods 1–5 or processing method 7 as described in Chapter 3 of Annex IV of EU Commission Regulation No 142/2011 have been considered acceptable to process.
UCO is vegetable/seed/animal oil that has been used to cook foodstuffs. Annex 1 point 22 of Commission Regulation (EC) No 142/2011 describes UCO as catering waste and classifies it as a Category 3 waste product. UCO will be de‐moistured (de‐watered) and filtered. When sourcing UCOs, they will be studied on a per batch basis in order to assess the necessary applicable pretreatment processes (if any) such that they meet the specification criteria. UCO (catering waste) will not necessarily have been processed using any of the processing methods 1–5 or processing method 7, as described in Chapter 3 of Annex IV of EU Commission Regulation No 142/2011.
AF and UCO can be sourced and purchased throughout the entire world. Due to the structural organisation of BP, all sourcing (purchasing) of raw materials occurs on behalf of bpRR by the trading department (Trading and Shipping) based in London. This business entity BP Oil International Limited will be doing all the sourcing of AF and UCO and is a registered trader. BP Oil International Limited will only be sourcing material from approved establishments as listed under the list on the website of the European Commission ABP Approved Establishments. 8
3.2.2. BIOHAZ Panel assessment of the material to be treated
The raw materials to be processed for the production of renewable fuels are AF and UCO. The application exclusively focuses on ABP Category 3 materials as described in Article 10 of Regulation (EU) No 1069 of 2009. As mentioned by the applicant, AF will be derived from a variety of ABP and from different countries, including those outside the European Union (EU). There is provision for the importation of rendered fats for the production of renewable fuels in Annex XIV of Commission Regulation (EC) No 142/2011. The conditions are set out in Section 1 and Section 9 of the Chapter II of Annex XIV. Rendered fats must be processed using any of the processing methods 1–5 or processing method 7. In addition, they must come from an establishment or plant that is registered and approved by the CA of the third country, and which is on the list of such establishments and plants referred to in Article 30 of Commission Regulation (EC) No 142/2011. Health certification is also required.
The application states that UCO will not necessarily be processed using any of the processing methods 1–5 or processing method 7 before being used for the production of a renewable fuel. Although it is clearly specified that only Category 3 ABP will be used to produce renewable fuels with this method, in Section 2.1 of the application, it is mentioned that all UCOs are Category 3 animal by‐products, as per point 22 Annex I Commission Regulation (EC) 142/2011. In fact, point 22 only defines catering waste but it does not mention the risk category. It is in Article 10 (p) of Regulation (EC) No 1069/2009 where it is specified that Category 3 ABP includes catering waste other than as referred to in Article 8(f). This latter article declares as Category 1 ABP catering waste from means of transport operating internationally. Therefore, catering waste could be Category 1 or Category 3 ABP. Only Category 3 UCO must be used to produce renewable fuels with the proposed method.
It is not clear if UCO can be imported into the EU for the production of renewable fuels as it is not specifically listed as one of the raw materials that can be imported into the EU for use outside the feed chain.
UCO is subjected to a high temperature when it is being used as a cooking oil. This is not the case for AF. However, in contrast with UCO, AF is always pretreated with methods 1–5 or method 7.
3.3. Hazard identification
3.3.1. Hazard identification as provided by the applicant
The microbiological hazards that could remain in AF are pathogenic bacteria that may include non‐spore‐forming bacteria such as Salmonella and other pathogenic Enterobacteriaceae and spore‐forming bacteria such as C. perfringens, although a wide range of possibilities exists because various points of origin (throughout the world) of the material exist.
As Category 3 fats (AF) that bpRR receives are already rendered following one of the standard methods 1–5 or 7 as described in Annex IV of Regulation (EC) No 142/2011, the probability of remaining bacteriological contamination is low.
The microbiological standards set out in Chapter I of Annex X of Regulation (EU) No 142/2011 do not apply to rendered fats and fish oil from the processing of ABP, when the processed animal protein, which is obtained during the same processing, is subject to sampling to ensure compliance with those standards. These standards are only required for derived products that are to be used as feed materials.
UCOs are vegetable/seed/animal oils and fats that have been used to cook or fry foodstuff (products of animal origin) for human consumption. Frying processes are carried out at temperatures of between 140–200°C. bpRR considers the remaining bacteriological risk in UCOs low due to the following reasons:
The cooking oils and fats have already undergone various manufacturing processes before being used as a medium for cooking foodstuffs.
As these oils and fats are used to cook meat or other products fit for human consumption, the exposure of humans to biological hazards is not expected.
The frying processes are carried out at temperatures of between 140–200°C.
Cross‐contamination may occur at various stages of the supply chain and can be caused by several different factors. The most likely occurrence of cross‐contamination would occur during transportation or storage with raw materials or contaminated consignments. Within normal transport or storage circumstances, the probability of cross‐contamination is very low.
Once on site, due to the configuration of the refinery and the pipelines from the tanks, the likelihood of microbiological or chemical cross‐contamination into other pipelines or tanks is negligible. Effectively, the materials from supply tanks and process installations are fed with pumps via pipelines to a header (a connection point that ties all the pipelines together) and arrive then to the initial drums (D806 and D2301 in GOH1 and GOH3, respectively) of the installation. Due to pump configurations, the flows are in one direction and the installation is designed such that the materials arrive together at the feed drums.
Given the possibility of the presence of various pathogens, depending on source and location, the assumption on the part of bpRR is to use the highest thermal resistance spore‐forming bacteria and base any further arguments on risk reduction techniques and factors (including pressure, temperature and exposure time) based on the characteristics of those with the highest resistance. Within the research provided, two different organisms are recognised: B. subtilis and Desulfotomaculum kuznetsovii spores. According to the applicant, B. subtilis spores have been recognised as the most heat resistant and have been known to survive extreme heat. This has been reported in at least two different papers (Molin and Snygg, 1967; Berendsen, 2016).
Bacillus subtilis is a Gram‐positive bacterium, rod‐shaped, catalase‐positive and has been known to survive extreme environmental conditions of temperature and desiccation. It is often considered the Gram‐positive equivalent of Escherichia coli. Bacillus subtilis has been granted ‘Qualified Presumption of Safety’ status by the European Food Safety Authority (EFSA BIOHAZ Panel, 2022).
While it is recognised that B. subtilis (and substances derived from it) have been evaluated by different authoritative bodies and generally recognised as safe, for this evaluation, some of the characteristics, namely the characteristics associated with the heat resistance, have been used to assess the estimated level of risk reduction.
Further investigations regarding heat‐resistant spores found a thermophilic species of D. kuznetsovii, a thermophilic, rod‐shaped, spore‐forming, sulfate‐reducing bacterium (Goorissen, 2002). In this particular study, approximately 10% of the spores of this organism survived a heat treatment at 140°C for 15 min that was considered ‘unprecedented’ by the authors. An analysis has also been carried out using this particular study.
With these choices, the purpose would be to prove that if there is sufficient risk reduction using the characteristics of this bacterium, then it would be equally applicable for existing pathogens with inferior heat resistance characteristics.
3.3.2. BIOHAZ Panel assessment of the hazard identification
According to the applicant, taking into account that AFs and UCO of very different origins can be used as feedstock, a wide range of biological hazards may be present in the material to be treated. Regarding AF, only rendered fats categorised as Category 3 and pretreated with methods 1–5 or method 7 as described in annex IV of Regulation (EC) 142/2011, will be used as raw materials, which reduces the likelihood of extensive contamination with hazardous biological agents. Similarly, for UCO, this material originates in restaurants, catering facilities and kitchens as a by‐product of the cooking at high temperatures of foodstuffs for human consumption, therefore the likelihood of extensive contamination with biological hazards is also reduced.
The applicant did not perform a full hazard identification process detailing all the relevant biological hazards for human and animal health related to the origin and category of the material to be processed. Instead, a few biological hazards that may be present in the material to be treated were identified, specifically mentioning Salmonella, other pathogenic Enterobacteriaceae and spore‐forming bacteria such as C. perfringens. Then, the applicant retained bacterial spores as the primary target for demonstrating the risk reduction achieved by the process, considering their high heat resistance. In particular, spores of B. subtilis and D. kuznetsovii were considered by the applicant as the primary target to demonstrate the ability of the method for hazard reduction. The approach followed by the applicant is consistent with one of the possible scenarios accepted: the selection of spores from non‐pathogenic spore‐forming indicator bacterial species as a primary target to demonstrate a sufficient level of hazard reduction, considering that any process achieving a significant level of inactivation of them will ensure a sufficient level of reduction of any more heat sensitive biological hazard that may be present in the Category 3 material.
Although the heat resistance of spores can vary significantly between bacteria species and even between strains of one species (Lima et al., 2011; Berendsen et al., 2015), B. subtilis is extensively used as a Gram‐positive model microorganism to understand sporulation and spore resistance mechanisms in aerobic spore‐forming bacteria (Wells‐Bennik et al., 2016) and can be considered a valid indicator for B. cereus and other Bacillus spp. Regarding D. kuznetsovii, it is not commonly used as an indicator microorganism, likely due to its extreme heat resistance, which greatly exceeds that of spores from all pathogenic bacterial species and is therefore not representative, but it can serve for the purpose of demonstrating the safety of the alternative method.
Chemical and physical hazards may also occur in the material to be treated, but are not addressed in this assessment.
3.4. Level of risk reduction
3.4.1. Level of risk reduction as provided by the applicant
To determine whether an acceptable level of risk reduction will be met, various scientific studies have been assembled. Although none of these studies modelled the exact conditions of bpRR's existing temperatures, pressures and retention times, the scientific literature assembled demonstrated an overall trend whereby when temperature is increased (and was kept constant for a period of time), the microbiological hazards in question were reduced in quantity (to various orders of magnitude). The scientific literature presented is meant to establish several arguments:
The microbiological hazards identified will follow the same behavioural pattern as the micro‐organisms presented in the scientific literature.
The environmental conditions (specifically temperature and pressure and retention time) in the scientific literature are less rigorous than those experienced in the hydrofiners at bpRR.
The temperatures and pressures to which the microbiological hazards are exposed at bpRR will provide equal or greater levels of risk reduction (and destruction of microbiological hazards) as those of the presented studies.
3.4.1.1. Scientific literature and reviews
Molin and Snygg (1967) demonstrated the heat resistance (and survival rate) of various bacterial spore types (B. megaterium, B. subtilis, B. cereus, B. stearothermophilus and C. botulinum type E) in various lipids including olive oil, soybean oil, triolein (a triglyceride) and liquid paraffin (the substance most likely to resemble LGO sent to the hydrofiners). The triolein and the liquid paraffin most likely to resemble the mixture of LGO, UCO and AF sent to the hydrofiners. The temperature at which the heat resistance was measured was between 80 and 121°C. In all cases, smaller D‐values at higher temperatures were reported. The study characterised the percentage of spores that survive at 112 and 121°C, respectively, and showed in all cases (to various degrees) the effect of temperature as well as exposure time. The percentage of spores that survives decreased and for liquid paraffin for B. cereus at 121°C (was reduced) to less than 0.1% after 30 min of exposure time. The study also further demonstrated the effects of adding water in various amounts to the oil and showed the percentage of surviving spores. The surviving spores in triolein also diminished in varying degrees although the introduction and effects of water (humidity) showed a decrease in resistance. Although the conditions in the study are not exactly representative of the conditions in the hydrofiners at bpRR, they demonstrate the effects of temperature and exposure time on spore‐forming bacteria.
Additional literature examples were sought for comparison. In the study by Ramirez‐Lopez (2006) the D‐value and Z‐value for thermoresistant bacteria subjected to thermal treatments at 91, 94 and 96°C are calculated. The D‐values in this case also show a remarkable decrease in time when the temperature was increased from 91 to 96°C. With the measurements that are made, a correlation is established in which the Z‐value in this study was calculated to be 17.68 ± 0.5429°C, meaning that for every 17.68°C, there was a 1 log order decrease in the remaining heat‐resistant bacteria, including spore‐forming bacteria. A further trend line is produced that relates the temperature to the log D‐values.
Berendsen (2016) compared two different methods of heating inactivation of 14 strains belonging to the B. subtilis group in which samples were studied in a batch treatment in capillary tubes and continuous flow heating in a microheater. The study shows a significant difference in both the D‐value and Z‐value for batch and continuous treatments in which the Z‐value for continuous flow treatment for high spore heat resistance was as high as 18.3 ± 2.2°C. The D‐values for batch and continuous flow treatments are also described and for high spore heat resistance were 45.7 s for D120°C but also indicated a decrease in time necessary for log order reduction as the temperature was increased.
Wijnands et al. (2009) calculated the D‐value and Z‐value for the spore‐forming bacterium C. perfringens in phosphate‐buffered saline at three different temperatures, from five strains of bacteria isolated from food. The highest Z‐value calculated was 14.31°C for strain Cp 5 (with the experiment carried out at a temperature range between 45 and 55°C).
As further supporting documentation, an ‘Evaluation of alternative methods of tunnel composting’ (EFSA BIOHAZ Panel, 2020b) provides both D‐values and the inactivation conditions for various pathogens (including Salmonella and C. perfringens). The D‐values if compared in the same medium, indicated that an increase in temperature (between 5 and 7.5°C) was associated with a large reduction in time for their respective D‐values. The inactivation for C. perfringens enterotoxins is stated at 60°C for 5 min. The inactivation temperature and time relation for Salmonella was also studied at temperatures ranging from 50 to 70°C, whereby the inactivation time was reduced substantially (from hours to minutes) in various media.
Studies regarding the effects of pressure on spore‐forming bacteria were also taken into account. Mills et al. (1998) found no significant inactivation when spores of Clostridium sporogenes were exposed to 600 MPa (6,000 bar) for 30 min at 20°C. When spores were exposed at 400 MPa (4,000 bar) and 60°C for 30 min at a combined pressure and heat treatment, this resulted in less than a 1 log10 reduction. Pressure cycling (e.g. 60 MPa followed by 400 MPa at 60°C) also reduced spore numbers although this resulted in less than a 3 log10 reduction.
Reddy et al. (2003) studied the effects of high‐pressure treatments at various temperature and time combinations on the inactivation of spores of C. botulinum type A strains 62‐A and BS‐A in phosphate buffer and a crabmeat blend. A 2 and 3 log10 reduction for BS‐A and 62‐A were, respectively, observed at a temperature/pressure combination of 827 MPa (8,270 bar) and 75°C for a processing time of 20 min in phosphate buffer. When processing for 15 min at the same temperature and pressure in crabmeat, the results were a reduction of 3.2 and 2.7 log10 units for BS‐A and 62‐A, respectively. Reddy and colleagues indicated that log10 unit reductions of spores increased significantly as processing time was increased from 5 to 20 min and pressure was increased from 414 MPa to 827 MPa and, in addition, no surviving spores were detected in phosphate buffer, carrot broth or meat broth following treatments of > 800 MPa at 80°C for 5 min.
Black et al. (2007) provided a comprehensive review of multiple studies regarding the effect of high‐pressure processing. In the review, Black and colleagues indicated that several studies have compared the efficacy of inactivation of bacterial spores by pressure at ambient temperature with that at higher temperatures. Elevation of pressure‐processing temperatures from ambient to > 50°C enhanced the inactivation of spores of Bacillus and Clostridium species. In addition, combining heat and pressure simultaneously or sequentially was more effective than pressure without heat.
Brown (2000) highlights some different bacterial spores including C. botulinum, C. perfringens, B. cereus, B. subtilis, B. licheniformis, C. butyricum, C. beijerinckii, C. pasteurianum, C. sporogenes, B. sporogthermodurans, C. thermosaccharolyticum, D. nigrificans, B. stearothermophilus, B. coagulans, Alicyclobacillus acidoterrestric and C. putrefaciens. According to the study, the most dangerous food poisoning species is C. botulinum whereas the most common are C. perfringens, B. cereus, B. subtilis and B. licheniformis.
Of particular interest in this study are some of the characteristics that were cited. Clostridium thermosaccharolyticum had some of the highest heat‐resistant spores with D121°C values as high as 68 and 195 min. Under dry heat conditions, spores of B. subtilis had extremely resistant D160°C values of 0.1–3.5 min, whereas D. nigrificans also displayed D121.1°C values as high as 55 min.
Further investigation into C. thermosaccharolyticum, later renamed to Thermoanaerobacterium thermosaccharolyticum, led to a study by Enache and Podolak (2013) in which they obtained the same results and cited that their Z‐values were between 6 and 7°C and also demonstrated a resistance to pressure‐assisted thermal processing (PATP).
Along with the C. thermosaccharolyticum information, Goorissen (2002) studied the characteristics of D. kuznetsovii, finding that approximately 10% of the spores of this organism survived a heat treatment at 140°C for 15 min that was considered unprecedented. This characteristic led to a thermal inactivation coefficient (Z‐value) of 16.7°C.
3.4.1.2. Analysis I
The scientific studies and literature that have been provided conducted a wide variety of experiments in various combinations of temperatures, pressures and for different lengths of exposure time with the purposes of characterising the conditions for the destruction of pathogens and describing the thermal resistance of spore‐forming bacteria.
Each study establishes a level of reduction in a target microbial hazard for a given time–temperature combination. The risk reduction level achieved varied considerably. When comparing the conditions to which the microbiological activity was exposed in all the studies with the conditions of the processes of bpRR, the maximum temperatures in the bpRR process are considerably higher (at certain times in excess of three times the temperature compared with those reported in the literature) whereas the pressures (when not measured under atmospheric conditions) were below those of the experiments that were conducted. Table 1 describes a summation of each component in each hydrofiner along with their respective pressures, temperatures and calculated minimum retention time to which AF and UCO will be exposed.
Based on the existing scenarios provided, in the environments described in both hydrofiners the temperature exceeds 270°C and pressures exhibited was at least 60 barg for at least 4.7 min (282 s). What this means is that, in all cases, any feedstock sent to either of the hydrofiners will experience the aforementioned conditions and they are considered the minimum exposure requirements.
A timeline is provided (Figure 2) to demonstrate the various temperatures to which the feedstocks will be exposed. A retention time of 30 s has been assumed for the T801 and T2301 stripper towers to show the temperature distribution. This retention time is only to demonstrate the temperature distribution across the strippers and is not used in any further analysis.
Figure 2.
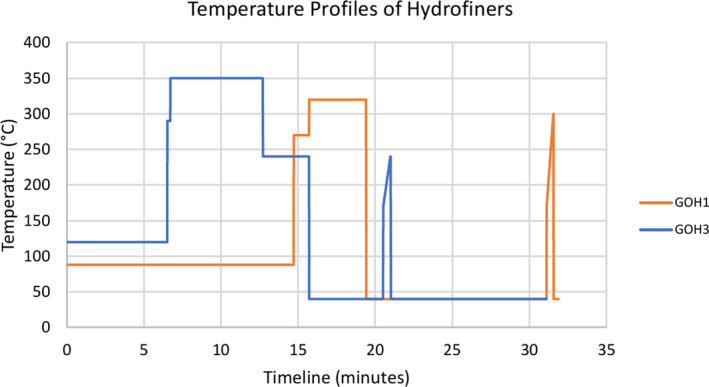
The timeline and temperature profiles of GOH1 and GOH3
Within the literature that was provided, the maximum temperature was 120°C and did not approach the temperatures at which the hydrofiners operate. To better understand and approximate the effectiveness and reduction in risk from the hydrofiners due to the temperature and retention time, an extrapolation technique with a correction to the formula was used to calculate the degree of risk reduction that would occur in the hydrofiners.
The extrapolation technique chosen was based on the available data, equations, and D‐ and Z‐values provided by Ramirez‐Lopez (2006). A Z‐value of 17.67°C was calculated by Ramirez‐Lopez in the study and is considered already a conservative number because it requires a larger temperature difference necessary to achieve a reduction in spore‐forming bacteria. However, the study claims that D‐values in foods with high fat content have been reported to be four to eight times higher than in a low‐fat medium. As such, a correction factor has been incorporated into the equation ■■■■■. A new Z‐value and equation was calculated based on the correction factor. With incorporating the characteristics of a high fat content (i.e. ■■■■■ the given D‐value from the Ramirez‐Lopez study), bpRR believes that it not only incorporates the possibilities of various AF and UCO compositions, but that this also would act as a conservative criterion because it requires an even larger temperature difference as well as more time to achieve a log reduction in spore‐forming bacteria.
In the study, Ramirez‐Lopez (2006) establishes a relationship between the log D‐values and different temperatures with the equation:
with x being the temperature (°C) and y at the log D‐value. This equates to a Z‐value of 17.67°C in order to reduce the spore‐forming bacteria by 1 log10 order of magnitude (as described by Ramirez‐Lopez, 2006).
With the correction factor and based on the original data provided by Ramirez‐Lopez (2006), a new D‐value and Z‐value were calculated (Table 2).
Table 2.
Original data from Ramirez‐Lopez (2006) and corrected D‐values
| Replication | Temperature (°C) | D‐values (min) | ■■■■■ |
|---|---|---|---|
| 1 | 90.76 | 54.88 | ■■■■■ |
| 2 | 91.02 | 52.24 | ■■■■■ |
| 3 | 90.22 | 67.75 | ■■■■■ |
| 1 | 94.77 | 65.15 | ■■■■■ |
| 2 | 94.54 | 43.81 | ■■■■■ |
| 3 | 94.94 | 53.91 | ■■■■■ |
| 1 | 95.8 | 24.14 | ■■■■■ |
| 2 | 95.98 | 22.89 | ■■■■■ |
| 3 | 95.98 | 27.51 | ■■■■■ |
■■■■■
■■■■■
Using this equation and extrapolating to a temperature of 270°C (the minimum operating temperature of the hydrofiner), Table 3 shows the reduction in log D‐values over the temperature range.
■■■■■.
■■■■■
| ■■■■■ | ■■■■■ | ■■■■■ |
|---|---|---|
| ■■■■■ | ■■■■■ | ■■■■■ |
| ■■■■■ | ■■■■■ | ■■■■■ |
| ■■■■■ | ■■■■■ | ■■■■■ |
| ■■■■■ | ■■■■■ | ■■■■■ |
| ■■■■■ | ■■■■■ | ■■■■■ |
| ■■■■■ | ■■■■■ | ■■■■■ |
| ■■■■■ | ■■■■■ | ■■■■■ |
| ■■■■■ | ■■■■■ | ■■■■■ |
| ■■■■■ | ■■■■■ | ■■■■■ |
| ■■■■■ | ■■■■■ | ■■■■■ |
| ■■■■■ | ■■■■■ | ■■■■■ |
| ■■■■■ | ■■■■■ | ■■■■■ |
| ■■■■■ | ■■■■■ | ■■■■■ |
| ■■■■■ | ■■■■■ | ■■■■■ |
| ■■■■■ | ■■■■■ | ■■■■■ |
| ■■■■■ | ■■■■■ | ■■■■■ |
| ■■■■■ | ■■■■■ | ■■■■■ |
| ■■■■■ | ■■■■■ | ■■■■■ |
| ■■■■■ | ■■■■■ | ■■■■■ |
| ■■■■■ | ■■■■■ | ■■■■■ |
| ■■■■■ | ■■■■■ | ■■■■■ |
| ■■■■■ | ■■■■■ | ■■■■■ |
■■■■■
Table 3 also demonstrates that the residence time of each reactor (GOH1 being 4 min and GOH3 being 6 min) should prove sufficiently long enough at the current operating temperature (being at a minimum but more than 270°C). Any temperature more than 160°C requires less than a few seconds to ensure a large risk reduction has been achieved.
Given that the studies regarding the effects of pressure were conducted at pressures ranging between 60 to greater than 800 MPa (600–8,000 bar), these pressures far exceed any of the pressures that are exhibited within the hydrofiners. Although Black et al. (2007) further discusses the combinations of both simultaneous and sequential heat and pressure treatment scenarios, those studies are also conducted with pressures that are greater than those of the hydrofiners and temperatures that are below the conditions within the hydrofiners. Within the Mills et al. (1998) and Reddy et al. (2003) studies, the reduction quantity purely from the pressure application varies between 1 and 3 log10 and is relatively small when compared with the extrapolated log10 reduction value from the corrected Ramirez‐Lopez equation. From the studies, it can be concluded that pressure will not have an adverse effect on the inactivation of spore‐forming bacteria but will have a minimal added effect for inactivation (less than 1 log10 order reduction, given that the magnitude of the pressure is considerably lower compared with the studies). Given the comparatively low level of log10 reduction for pressure, the minimal added effects of the pressure were not incorporated into the extrapolated amount of risk reduction within the reactors; however, in order to facilitate the chemical reaction within the hydrofiners, pressure should still be (and still is) considered an important factor (critical control point) to produce renewable fuels.
Once this step is completed, any spore‐forming bacteria are not expected to be present after passing through the reactors based on the reduction in logarithmic values. However, within the existing configuration of the hydrofiners, all the products must go through the stripper towers for further distillation.
The same methodology can be applied to the stripper section of the hydrofiners, in which the mixture of AF, UCO and LGO, once cooled to 40°C after the reactors and separators, is reheated at the entrance of the stripper to 170°C and travels through the strippers to a temperature of 240°C (for GOH3) and 300°C (for GOH1). ■■■■■ Although this second step provides a lower reduction of magnitude compared with the initial step, in the stripper, steam is introduced that provides a different environment than the conditions within the reactors. As observed by Molin and Snygg (1967), the introduction of humidity into the experiments resulted in a decrease in resistance by spore‐forming bacteria and that should be applicable for the strippers as well. With steam injected at the bottom of the stripper and travelling upwards through the tower, and LGO, UCO and AF entering and travelling down the tower, steam is expected to come into contact with the products. With the pressures of each stripper being relatively low, the risk reduction arising from the pressure is considered negligible.
3.4.1.3. Analysis II – extrapolation 2
Within the supplemental studies provided, Goorissen (2002) brings together numerous strains of bacteria with their respective characteristics. Specifically, Table 4 within the study highlights the highest reported D120 values. This table includes the earlier cited strains C. thermosaccharolyticum as well as D. nigrificans. However, it concentrates on D. kuznetsovii showing extremely heat‐resistant spores with a D140 of 15 min. At lower temperatures, the D‐values are extremely high (D130 = 79.2 min, D120 = 40 min 9 ) and have an associated Z‐value of 16.7°C. With the equation:
Table 4.
Extrapolation of log D‐values of the Goorissen equation to 270°C
| ■■■■■ | ■■■■■ | ■■■■■ |
|---|---|---|
| ■■■■■ | ■■■■■ | ■■■■■ |
| ■■■■■ | ■■■■■ | ■■■■■ |
| ■■■■■ | ■■■■■ | ■■■■■ |
| ■■■■■ | ■■■■■ | ■■■■■ |
| ■■■■■ | ■■■■■ | ■■■■■ |
| ■■■■■ | ■■■■■ | ■■■■■ |
| ■■■■■ | ■■■■■ | ■■■■■ |
| ■■■■■ | ■■■■■ | ■■■■■ |
| ■■■■■ | ■■■■■ | ■■■■■ |
| ■■■■■ | ■■■■■ | ■■■■■ |
| ■■■■■ | ■■■■■ | ■■■■■ |
| ■■■■■ | ■■■■■ | ■■■■■ |
| ■■■■■ | ■■■■■ | ■■■■■ |
| ■■■■■ | ■■■■■ | ■■■■■ |
| ■■■■■ | ■■■■■ | ■■■■■ |
| ■■■■■ | ■■■■■ | ■■■■■ |
This data point provided by the applicant in the dossier is not correct. It should be 0.181.
an extrapolation from 120 to 270°C can be made and is provided below in Table 4.
As a result, using the extrapolation methodology, from 120 to 270°C, any spore‐forming bacteria would be reduced by a ■■■■■ (calculated by the difference in log values between 270 and 120°C). The equivalent time has been calculated based on the logarithmic value. As with the previous result from the modified Ramirez‐Lopez equation, the equation taken from Goorissen also demonstrates a similar result.
3.4.1.4. Analysis III – sensitivity model
The presented studies focus on studying the D‐ and Z‐values of various pathogens. A sensitivity analysis (parametric modelling) has been incorporated into this application to further examine the risk reduction conditions around Z‐values. The purpose of this analysis is to understand the relationship of the existing process conditions and model various Z‐values against the process temperature conditions and then to compare the theoretical logarithm reduction versus the minimum operating temperature.
This analysis is not related to any study, but assumes a linear relationship between the heat kinetics and risk reduction. To date, no Z‐values have exceeded 20°C. However, this analysis explores four different Z‐values that were applied across the temperature range (from 90 to 270°C). The Z‐values chosen are 18, 20, 25 and 30 and the log10 orders are listed Table 5.
Table 5.
Various theoretical Z‐values applied across the temperature range of the hydrofiners
| ■■■■■ | ||||
|---|---|---|---|---|
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ |
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ |
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ |
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ |
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ |
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ |
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ |
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ |
| ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | |
| ■■■■■ | ■■■■■ | ■■■■■ | ||
| ■■■■■ | ■■■■■ | ■■■■■ | ||
| ■■■■■ | ■■■■■ |
In this analysis, it is demonstrated that, when using a Z‐value of ■■■■■ applied over the most conservative temperature range of the processes, ■■■■■ would take place. This temperature would be applied for a minimum retention period of 4.7 min (282 s) for all process conditions.
3.4.1.5. Analysis IV – retention time
The retention time through both process units is also an aspect that has been investigated further. To better understand the risk reduction associated with retention time, research data were reviewed and modelled (or applied) to the existing retention time of the hydrofiners. The analysis studied various D‐values. The selected D‐values are based upon several criteria: heat resistance data of the B. subtilis and D. kuznetsovii strains provided by Berendsen (2016) and Goorissen (2002); high Z‐values within the provided research (> 15°C)
The associated data in Table 6 were provided by Berendsen and use the associated D‐value of a high Z‐value result. The temperatures are also at the higher end of the spectrum within the data provided and, in the last data set, the larger D‐value between the batch and flow data set was used as a conservative measure. Although the heating method was a batch mode, whereas the flow heating method had a much higher Z‐value, the associated D‐value was 8.5 s (also at the same temperature). As a check, from the same study in Table 6, similar D‐values were spotted for strain 4,067 for which at 125°C the respective D‐value was 0.53 min (31.8 s) and for strain 4,069 for which at 120°C the respective D‐value was 0.58 min (34.8 s). The D‐values are shown in minutes and seconds and a calculation has been made that indicates the number of iterations that would occur within 4.7 min (282 s – the minimum time that UCO and AF would experience a temperature of 270°C) whereby the strains would be reduced by 1 log10 factor.
Table 6.
Various theoretical Z‐values applied across the temperature range of the hydrofiners
| Z‐value (°C) | Temperature (°C) | D‐value (min) | D‐value (s) | ■■■■■ |
|---|---|---|---|---|
| 15.57 | 125 | 0.53 | 31.8 | ■■■■■ |
| 15.84 | 120 | 0.58 | 34.8 | ■■■■■ |
| 18.3 | 120 | 0.76 | 45.7 | ■■■■■ |
In the most conservative calculation and at a temperature of 120°C (far below the operating temperatures of the hydrofiners), at 282 s, the reduction factor was greater than 6 log10.
One further analysis regarding the retention time was made regarding the data provided by Goorissen (2002) for D. kuznetsovii. The D‐values are provided in Table 7.
Table 7.
Summarised D‐values for D. kuznetsovii
| Temperature (°C) | D‐value |
|---|---|
| 90 | 11 days |
| 100 | 70 h |
| 120 | 240 min |
| 130 | 79.2 min |
| 140 | 15 min |
In this analysis, a calculation/extrapolation is made to ascertain at what temperature for a time of 282 s the reduction factor would exceed 6. From the earlier formula, the temperatures have been extrapolated and displayed in Table 8.
Table 8.
Extrapolated D‐values and associated reduction factors for D. kuznetsovii
| Temperature (°C) | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ |
|---|---|---|---|---|
| 159 | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ |
| 160 | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ |
| 161 | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ |
| 162 | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ |
The reduction factor in Table 8, while based on extrapolated data, is not based on the Z‐values but rather a retention time reduction value whereby the temperature at 161°C exceeds six factors of time, based on the risk reduction cycles when exposed for 282 s. The temperature of 161°C is more than 100°C lower than the minimum temperature of the hydrofiners.
3.4.1.6. Risk reduction factors
There are several different methods that bpRR will use to reduce the level of risk of the material that will be processed to both people and the environment.
Before the feedstock is accepted on site for processing, it will be ensured that AF derived from ABP is first processed using any of the processing methods 1–5 or processing method 7, as described in Chapter 3 of Annex IV of EU Commission Regulation No 142/2011 and UCO will comply with the definition by Annex 1 point 22 of Commission Regulation (EC) No 142/2001.
Given that the materials (AF and UCO) that will be brought on site for processing all fall under Category 3 material, which has been assessed as a low risk, then all possible remaining pathogens (the probability of remaining contamination is low), including Salmonella, Enterobacteriaceae and spore‐forming bacteria such as C. perfringens are expected to be inactivated by the hydrotreatment process.
3.4.1.7. Inactivation
Inactivation of pathogens is mainly driven by the effects of high temperatures; however, the effects of pressure and retention time in two different sections of the process installations are also important.
The first section at which inactivation will take place is within the reactors of each hydrofiner. The reactors provide the harshest environment within the entire process with respect to temperature and pressure. The pressure within the furnace, reactors, and separators for both hydrofiners is greater than 60 barg. Although the temperature varies in different sections of each piece of equipment of each unit, within the reactors of each hydrofiner the temperature is always greater than 270°C.
The second section at which any further inactivation will take place (if any spore‐forming bacteria remain) is within the strippers of each hydrofiner. With the introduction and exposure to steam of the product, with temperatures always more than 170°C and a pressure of 5.5 barg, this environment will eliminate any remaining presence of spore‐forming bacteria.
Should the temperature or pressure drop out of the specified range, the reactions will not take place and the end products will not meet specification. Should such an instance occur, the hydrocarbons will be sent to a tank and rerouted for proper processing. The retention time has been calculated based on the flow rates and size of the equipment. It is not monitored. The installation is continuously monitored in the distributed control system (DCS) system.
3.4.1.8. Level of risk reduction
The level of risk reduction occurs at two different sections within each hydrofiner: the reactor and the stripper. While the environment of the reactor is the harshest with respect to temperature and pressure, the stripper introduces steam into the system that will also have an adverse effect on any remaining heat‐resistant bacterial spores.
As most of the studies were conducted at temperatures ranging between 80 and 120°C, an extrapolation was used based on the data provided by Ramirez‐Lopez (2006), whereby the Z‐values and the associated equation provided allowed for a level of risk reduction to be calculated. This technique was applied to both the reactor and the stripper, even though the environments are different from each other.
■■■■■
The operating pressure of the reactor and the stripper will provide an extra level of risk reduction although relatively small (negligible) compared with the risk reduction of the elevated temperatures.
When assessing the retention time, one of the general observations that can be made from all the literature and studies is with higher temperatures, the D‐values decrease, meaning that it takes a shorter amount of time to reach the same level of inactivation. ■■■■■ Given that the retention time within the reactors of the hydrofiners is in minutes, the retention time should be adequate to achieve a sufficient level of risk reduction.
Although the retention time within the stripper is unknown, the necessary time (greater than several seconds) within the stripper should be achievable given the temperature range (170–240°C) that the strippers exhibit. Due to the configuration of the strippers (a tower with numerous trays that allows steam to pass up and product to travel downwards), bpRR believes it is reasonable to assume that the retention time is sufficient for ■■■■■ to effectively take place.
Further analysis has been provided that supports an adequate level of risk reduction. In Section 3.4.1.3, another extrapolation was used (provided) by Goorissen (2002). Using the extrapolation technique, a reduction ■■■■■.
In Section 3.4.1.4, a sensitivity analysis modelled various Z‐values from 90 to 270°C. In the most conservative case in which a theoretical Z‐value of 30°C was chosen and applied over the range, a ■■■■■ order reduction was seen.
A further retention time analysis was carried out using data provided by Berendsen (2016) whereby at the provided temperature of 120°C, and a D‐value of 0.76 min (45.7 s), the time reduction factor (the number of log order reduction cycles) is ■■■■■ when applied for 4.7 min.
Lastly, an analysis was carried out using Goorissen's data by extrapolating the temperature using the provided equation to ascertain at what temperature for 4.7 min a reduction factor of log10 order would exceed 6. The temperature for this case would be 161°C (far below the minimum operating temperature of the reactors of the hydrofiners).
As a result, the final product (renewable fuels – gas oil) is not expected to contain any spore‐forming heat‐resistant bacteria and, considering the nature of the final product, a very low level of risk for humans and animals associated with the intended end use is expected.
3.4.2. BIOHAZ Panel assessment of the level of risk reduction
The applicant, as already mentioned in the hazard identification section, did not perform a specific hazard identification exercise but used spores from non‐pathogenic indicator bacterial species as a primary target to demonstrate a sufficient level of risk reduction (Section 3.3.2).
In the scientific literature and reviews section, the applicant presents some studies related to microbial heat inactivation performed with different spore‐forming bacteria and substrates, and at different temperatures. A study by Berendsen (2016) compares the inactivation of 14 strains belonging to the B. subtilis group in a batch treatment (capillary tubes) and under continuous flow heating, where D120 values of 0.45 min were obtained and a reduction of times at higher temperatures was observed. Several studies mentioned provided evidence that, in lipid substrates, D‐value and Z‐value increase considerably (Molin and Snygg, 1967), supported also by others available in the scientific literature (Van Asselt and Zwietering, 2006).
Then, different analyses are provided. The first two (Analyses I and II) are based on extrapolations from the D‐value and Z‐value published for indicator microorganisms (with experimental values at temperatures of up to 120°C) to the temperature of the process, 270°C. The log reductions reported in such extrapolation analyses represent the log D‐value reductions as a result of an increase in treatment temperature to 270°C, rather than the log reductions in the population of the indicator microorganisms expected as a result of the alternative process. Although the D‐value and Z‐value can be used to estimate values at conditions different from the experimental ones, the risk of extrapolation has been indicated in the scientific literature. A recent study (Peleg, 2021) states that there is not enough information in an experimental survival curve's shape to allow its continuation to below the detection level. Therefore, any thermal death time determined by extrapolation can lead to underestimation or overestimation of the lethality of the thermal process. Masana and Baranyi (2000) also stated that the risk of extrapolation can become unexpectedly high during the extension of a model to describe the effect of newer factors, if the extension is supported by insufficient data. Therefore, data extrapolated beyond the interpolation region were not considered in the assessment. The only assumption considered valid is that, if a heat inactivation temperature provides enough level of risk reduction, higher temperatures will provide, at least, the same level of reduction.
Analysis III is a sensitivity analysis that is not based on actual data, but theoretical high Z‐values, and therefore the risk of extrapolation mentioned above would also apply. Analysis IV is based on the retention time and data from the literature in B. subtilis and D. kuznetsovii from Berendsen (2016) and Goorissen (2002), respectively. For D. kuznetsovii, a very high heat‐resistant microorganism, the level of reduction is calculated by extrapolation, therefore the limitation mentioned in the previous paragraph remains valid. For B. subtilis, an indicator microorganism for Bacillus spp., including the foodborne pathogen B. cereus, it was shown, based on experimental data, that a sufficient level of risk reduction would be achieved for spores of this indicator bacterium at lower treatment temperatures (> 5 log reduction after 4.7 min at 120°C) than those of the alternative method (Table 6). It can be assumed that the level of risk reduction achieved at 270°C will be, at least, the same as that obtained at those lower temperatures, following the criterion mentioned above.
Additionally, in a recent meta‐analysis of the heat resistance of C. botulinum and C. sporogenes (a non‐ pathogenic indicator), in total 911 D‐values collected from 38 studies were used (Diao et al., 2014). They showed average D‐values of 0.19 and 1.29 min at 121.1°C, respectively. At the highest temperature for which a D‐value was measured for C. botulinum (140°C), considering the upper 95% credibility interval boundary of the study, a D140 of approximately 0.01 min (−2 log10 D‐value) was reported. Therefore, considering a minimum retention time of 4.7 min, at temperatures of 140°C or higher, reductions in excess of 400 log10 of spores of the more heat‐resistant strains of C. botulinum would be expected. Even considering the possible increase in heat resistance due to the lipid substrate mentioned by the applicant, an inactivation that exceeds the requirements (12 log10 reductions for C. botulinum spores) would be expected. Again, the higher temperature applied (270°C) would provide, at least, the same level of risk reduction following the conservative approach suggested.
Therefore, considering the evidence presented and some additional evidence from the literature, even if the extrapolation analyses proposed by the applicant cannot be considered valid, the expected level of reduction achieved for the hazards that may be present is considered to be at least the reduction required by the standards indicated for Category 3 material (Section 2.2.2).
3.5. HACCP plan
3.5.1. HACCP plan as provided by the applicant
The HACCP plan has been devised based on identification of critical control points for bpRR's process. The HACCP plan was developed in accordance with Regulation No 1069/2009 and Regulation No 142/2011. Critical control points were identified based on the decision tree found in the Codex Alimentarius (Appendix B), and are presented in Table 9. Each step throughout the process was analysed according to the decision tree and it was determined whether it was considered a critical control point.
Table 9.
The hazard analysis critical control point identification process using the codex decision tree
| Process step | Hazard | Probability | Severity | Risk | Control measure | Decision tree | Corrective action/motivation |
|---|---|---|---|---|---|---|---|
| Import AF and UCO | Microbiological – existence of pathogens for entry to site | Small | Small | 1 | Pretreatment of AF and UCO confirmed with document control and Bill of Lading | Q1: Yes; Q2: Yes; → CCP | Material not accepted on site and discharge into storage does not take place. The material must be returned and reprocessed until it meets the specification of insoluble impurities |
| Preblending and storage | |||||||
| Preblending and storage | Microbiological contamination in tanks and or spill | Small | Small | 1 | Contained tank with level indicators and mixers | Q1: Yes; Q2: No; Q3: Yes; Q4: Yes; → Not a CCP | Initiate spill respond procedure, contain and clean spill. Repair storage tanks |
| Hydrofiner co‐processing steps | |||||||
| Feed system | Microbiological contamination – spill or failure of equipment | Small | Small | 1 | Contained system with automated and backup systems (pump) | Q1: Yes; Q2: No; Q3: Yes; Q4: Yes; → Not a CCP | The process will be suspended, equipment will be repaired (or replaced). The process will be restarted, and the cycle repeated |
| Heat exchangers | Microbiological contamination – spill or failure of equipment | Small | Small | 1 | Automated systems monitor and regulate flow rates | Q1: Yes; Q2: No; Q3: Yes; Q4: Yes; → Not a CCP | The process will be suspended, equipment will be repaired (or replaced). The process will be restarted, and the cycle repeated |
| Furnace | Microbiological contamination – spill or failure of equipment | Small | Small | 1 | Automated systems monitor temperatures and operating parameters of equipment | Q1: Yes; Q2: No; Q3: Yes; Q4: Yes; → Not a CCP | The process will be suspended, equipment will be repaired (or replaced). The process will be restarted, and the cycle repeated |
| Reactors | Microbiological – spill or failure of equipment | Small | High | 3 | Pressure and temperature (and retention time of the product) of equipment is monitored to ensure reactor remains within operating envelope (parameters) | Q1: Yes; Q2: Yes; → CCP | The process will be suspended, equipment will be repaired (or replaced). The process will be restarted, and the cycle repeated |
| Separators | Microbiological – spill or failure of equipment | Small | Small | 1 | Pressure and temperature of equipment is monitored to ensure separators remains within operating envelope (parameters) | Q1: Yes; Q2: No; Q3: Yes; Q4: Yes; → Not a CCP | The process will be suspended, equipment will be repaired (or replaced). The process will be restarted, and the cycle repeated |
| Product stripper | Microbiological – spill or failure of equipment | Small | Medium | 2 | Pressure and temperature (and retention time of the product) of equipment is monitored to ensure reactor remains within operating envelope (parameters) | Q1: Yes; Q2: Yes; → CCP | The process will be suspended, equipment will be repaired (or replaced). The process will be restarted, and the cycle repeated |
| Renewable fuel storage | |||||||
| Storage/export | Microbiological and chemical – off spec product | Small | High | 3 | Material sampling to ensure end product meets ISO/EN requirements (or equivalent) | Q1: Yes; Q2: Yes; Q3: No; → Not a CCP | Off spec material is sent for reprocessing |
Prerequisite programmes
The bpRR has numerous processes, procedures and conditions already in place including the following:
Acceptance criteria for all feedstocks (and products)
Operational procedures
Personal safety and hygiene procedures and requirements
Process Safety risk assessments and procedures
Material sampling procedures
Cleaning and laboratory procedures
Pest control procedures
Maintenance and calibration procedures
Internal Auditing
Emergency Response Procedures
Spill Response Plan
Ground Contamination Plan and response
Documentation and Control plans
Inspection Plans and procedures
Self‐verifications
Communication Procedures
Environmental Permits.
These procedures are part of the Operating Management System (OMS) and enable the ISO 9001 and 14,001 certifications. These processes, procedures and regulations help to define mitigative actions and techniques for critical control points.
It is important to note that numerous procedures and response plans have been designed and developed to ensure and to address possible adverse scenarios. In many cases, these response plans have been reviewed and approved by various government bodies and organisations (in some cases in which it is legally mandated).
CCP No. 1 material intake
The first parameter that must be checked is whether AF and UCO comply with the standards and specifications that bpRR has designated (Appendix 1). Category 3 AF must have already been processed by methods 1–5 or 7 as described in Chapter 3 of Annex IV of EU Commission Regulation No 142/2001, and the content of the insoluble impurities ■■■■■.
If the values are higher than the criteria as per the bpRR specification, the feedstock must be rejected, and the material must be returned and reprocessed until it meets the specification of insoluble impurities.
CCP No. 2a reactor temperature
In order to achieve thermal inactivation, the reactors must be operating within the specified temperature ranges. Should the temperature fall below the range, the reactions may not take place and the desired level of risk reduction will not be achieved. If such an event were to occur, the products would be diverted to the intermediate (slops) tanks and later be reprocessed. To ensure the temperature remains above critical values, it must therefore be monitored continuously.
CCP No. 2b reactor pressure
The pressure is created by a high‐pressure pump. The pressure is automatically controlled and needed for the reaction. If the pressure is below the limit, the chemical reactions will not occur. If such an event were to occur, the products would be diverted to the intermediate (slops) tanks and later be reprocessed. In addition, with the temperature being a function of the exothermic chemical reactions taking place, as well as pressures exerted throughout the installations, pressures are also critical control points in order to achieve the reactions.
CCP No. 2c retention time
Although the necessary retention time within the reactors in order to achieve an acceptable level of risk reduction is not very long, the exposure time is still an important factor that needs to be taken in account. A minimum retention of 3.7 min (plus 1 min including time in the furnace) based on the maximum flow rate conditions is deemed adequate.
The process parameters (temperatures and pressures and retention time, CCP No. 2a, 2b and 2c) are critical to achieve an acceptable level of risk reduction in the reactor for the inactivation of any pathogens described in Section 5.
CCP No. 3a stripper temperature
To ensure the risk reduction takes place as described in Section 6, the material must be exposed to the temperatures in the stripper tower. The temperature is monitored at various locations throughout the tower via the DCS system and is logged. Should the temperatures not be achieved, the products would be diverted to intermediate (slops) tanks and later be reprocessed.
CCP No. 3b stripper pressure
Similar to CCP No 3a. the pressure is also a requirement that guarantees that the process is under control and that this section ensures that the final renewable fuels (gas oil) are correctly being produced. Should the pressure not be achieved, the products would be diverted to intermediate (slops) tanks and later be reprocessed.
CCP No. 3c retention time
Even though the retention time within the strippers in order to achieve an acceptable level of risk reduction is not very long, the exposure time is still an important factor that needs to be taken into account. The important element regarding the retention time is not the length of time itself, but the successful entry and exit of the materials and exposure to the stripper's temperature and pressure.
To achieve an acceptable level of risk reduction within the stripping step, temperature and pressure (CCP No. 3a, 3b and 3c) are necessary.
The critical parameters (pressure and temperature) are controlled by process measuring and control devices. The DCS system allows for continual monitoring (and visualisation) of the system in which staff (operators) are on site 24 h/day to monitor the system. If specific process values are not met, the system shuts down and the unprocessed product is rerouted to a tank and cannot leave the closed system.
The results of the controls applied to the critical points will be kept for a minimum of 2 years. If during one of the checks that are carried out it is discovered that a critical point is not properly controlled, corrective measures shall be applied as soon as possible to resolve the situation.
Additionally, in case of modification of any stage in the process or the product, the control processes shall be reviewed and adapted when required. A management of change process (as described in the bp OMS) may be applied to ensure the change shall be handled properly.
3.5.2. BIOHAZ Panel assessment of the HACCP plan
The applicant sets a HACCP plan for the process taking place either in one or the other of the two gas oil hydrofiners (i.e. GOH3 and GOH1). GOH3 has a maximum design flow of 435 m3/h, and GOH1 of 220 m3/h. In GOH3, the feed system has a maximum operating temperature of 120°C, whereas in GOH1 it is 88°C. In GOH3, the furnace is equipped with six burners, whereas in GOH1 there are 16 burners. In GOH3, each reactor has two catalyst beds consisting of CoMo and NiMo (cobalt molybdenum and nickel molybdenum) catalysts, whereas in GOH1 only a CoMo catalyst is used to promote chemical reactions and desulfurisation. Differences in operating conditions and retention times of the equipment of GOH3 and GOH1 are reported in Table 1.
The applicant provides two tables dealing with the HACCP plan: Table 9, on the HACCP identification process; and Table 10 on the HACCP plan for AF and UCO on site at bpRR. The biological, chemical and physical hazards identified by the applicant as relevant in AF are described in the application and are summarised in Section 3.3.2.
Table 10.
Critical control points of the HACCP plan proposed by the applicant for the implementation of the new alternative method
| Reception of AF and UCO | |||||
|---|---|---|---|---|---|
| Flowchart | Type of hazard | Standard operating procedure | Critical control point | Monitoring procedures and devices | Corrective procedures |
| Reception of AF and UCO | Biological/Chemical | Laboratory test results to be reviewed and accepted prior to acceptance of material on site | Insolubilities and impurities defined in bp specifications must be below the limit ■■■■■ (Appendix 1) | Sample taken prior to receipt of material | If sample does not meet bp specification criteria, the ship will not be allowed to discharge material on site. The material must be returned and reprocessed until it meets the specification of insoluble impurities |
| Gas oil hydrofiner 1 (GOH1) | |||||
| Reactors – R801 and R802 | Biological | Leerboek – GOH1; D0302P01 – Optimiser | 1. Temperature: 320°C; 2. Pressure: 60 barg; 3. Retention time: 3.7 min (based on maximum flow in the unit) | Pressure indicator: PDL811; Thermocouple: TR801, THCO834; Pressure indicator: PICV801B | The process will be suspended, equipment will be repaired (or replaced). The process will be restarted, and the cycle repeated |
| Product Stripper T801 | Biological | Leerboek – GOH1 | 1. Temperature: 170°C; 2. Pressure: 7 barg; 3. Retention time: Entry and exit of AF and UCO while having been exposed to the given operating conditions | Level transmitters: LT810A, LT810B, LT810C; Level indicator: LIC810; Flow controller: FIC820; Pressure controller: PIC802 | The process will be suspended, equipment will be repaired (or replaced). The process will be restarted, and the cycle repeated |
| Gas oil hydrofiner 3 (GOH3) | |||||
| Reactors – R2301 and R2302 | Biological | Leerboek – GOH3; D0302P01 – Optimiser | 1. Temperature: 350°C; 2. Pressure: 65 barg; 3. Retention time: 6 min (based on maximum flow in the unit) | Thermocouple: THCO2395; Pressure indicator: PIC2340; Flow regulator: FIC2327, FIC2328, FIC2311 | The process will be suspended, equipment will be repaired (or replaced). The process will be restarted, and the cycle repeated |
| Product stripper – T2301 | Biological | Leerboek – GOH3 | 1. Temperature: 170°C; 2. Pressure: 5.5 barg; 3. Retention time: Entry and exit of AF and UCO having been exposed to given operating conditions | Temperature controller: TIC 2316; Temperature controller: TV 2303; Pressure indicator: PTS2305; Flow Valve: FV2307; Pressure controller: PICA2305 | The process will be suspended, equipment will be repaired (or replaced). The process will be restarted |
The first step identified among what the applicant describes as process steps in Table 9 and as a flow chart in Table 10 is named ‘Import of AF and UCO’ in Table 9 and ‘Reception of AF and UCO’ in Table 10. The hazards identified in that step are microbiological hazards, defined generically as pathogens, in Table 9, whereas in Table 10 both biological and chemical hazards are listed. The same name should be associated with the first step described in Tables 9 and 10. Moreover, the hazards associated with that step should be the same. In addition to differences in the definition of the first step, the reception of AF and UCO is identified by the applicant as a CCP. However, #6.1 of the dossier on the prerequisite programme lists the acceptance criteria for all feedstocks (and products) as part of the OMS. At the point of arrival, Category 3 AF must have already been processed by methods 1–5 or 7, as described in Chapter 3 of Annex IV of Commission Regulation (EU) No 142/2001, and the content of the insoluble impurities ■■■■■. As the acceptance of the material on site follows the analysis of samples taken before receipt of material, this is considered a prerequisite and not a CCP. When the values in the tested samples are out of the specifications, the feedstock must be rejected for processing and returned to the supplier for reprocessing until it meets the specifications of insoluble impurities. As far as UCO is concerned, it is de‐moistured and filtered. When sourcing UCOs, they are considered on a per batch basis to assess the need for pretreatments. However, it is not clear if this occurs before or after the acceptance of UCOs. In Table 10, critical limits should be listed for each hazard identified in a CCP, whereas for the reception of AF and UCO the applicant refers generically to insolubilities and impurities.
The second step in Table 9 is referred to as preblending/storage and refers to what happens in tanks 146, 149 and 151 with a storage capacity of 36,000 m3 each, and tanks 59 and 61 with a maximum capacity of 1,100 m3. In tanks 146, 149 and 151, AF and UCO are mixed with LGO to prevent coagulation, whereas in tanks 59 and 61 pure AF and UCO are stored, without mixing with LGO. This step is not identified as CCP and this is considered correct. A microbiological contamination can occur in tanks and/or spills, but this is prevented using level indicators and mixers and initiating a spill response procedure to contain and clean the spill and repair the storage tank in case of problems.
The following steps in Table 9 are identified as hydrofiner co‐processing steps (see Figure A.1 in Appendix A) and include: (1) feed systems (i.e. D2301, referring to GOH3, and D806, referring to GOH1) receiving AF and UCO with or without LGO; (2) heat exchangers; (3) furnace (H2301 and H801); (4) reactors (R2301 and R2302, and R801 and R802); (5) high‐pressure separators (D2302 and D2303, and D801 and D802); and (6) product strippers (T2301 and T801).
The feed systems, heat exchangers and furnace are not identified as CCPs and this is considered correct. A microbiological contamination might derive from spill or failure of the equipment. However, for the feed systems, the microbiological contamination is prevented by contained systems with automated and backup systems (pump) and eventually eliminated suspending the process and repeating the cycle. For the heat exchangers, microbiological contamination is prevented using automated systems, monitoring and regulating the flow rates, and is eventually eliminated, suspending the process and repeating the cycle. Finally, for the furnace the microbiological contamination is prevented using automated systems, monitoring the temperature and other operating parameters of the equipment, and eventually eliminated, suspending the process and repeating the cycle.
In furnace H2301, the temperature reaches 290–350°C for 0.2 min with a pressure of 65 barg, whereas in furnace H801, the temperature reaches 270–320°C for 1 min with a pressure of 60 barg. Although these temperature and pressure values in the furnaces for GOH3 and GOH1 are able to inactivate biological hazards, the reactors work at higher temperatures and for longer times. Therefore, the reactors are identified as CCPs and this is considered correct. The limits set by the applicant for these CCPs (Table 10) are clear and in line with the parameters listed in Table 1 for both GOH3 and GOH1.
According to the applicant, temperatures, pressures and flows are monitored and recorded continuously using a DCS. The DCS system is routed to the control room that is manned by operators that work in shifts. The facility and DCS system have warnings and alarms programmed to alert operators when conditions exceed predefined operating parameters. When temperature, pressure and retention time parameters do not reach the expected values the process is suspended, the products diverted to the intermediate tanks, and the equipment repaired or replaced, before restarting the process and repeating the cycle.
The high‐pressure separators are not identified as a CCP and this is considered correct. A microbiological contamination might derive from a spill or failure of the equipment. These events are prevented by monitoring pressure and time to ensure separators remain within the operating parameters. If these operating parameters do not reach the expected values, the process is suspended and equipment is repaired or replaced before restarting the process and repeating the cycle.
In both hydrofiners, the product stripper is identified as a CCP and this is considered correct. The limits set by the applicant for these CCPs (Table 10) are clear for both temperature and pressure and in line with the parameters listed in Table 1 for both GOH1 and GOH3. According to the applicant, the retention time of the liquid in the tower cannot be calculated due to the design of the tower including its trays. However, the critical step is the successful entry and exit of the materials and exposure to stripper temperature and pressure. Nonetheless, the mean of verification of the entry and exit steps is unclear.
For the reactors, for the product stripper the temperature and pressure values are monitored and recorded continuously using a DCS. When the temperature and pressure do not reach the expected values, the process is suspended, the products diverted to the intermediate tanks, and the equipment repaired or replaced, before restarting the process and repeating the cycle.
The last step of the process, represented by the renewable fuel storage and export (Table 9), is not identified as a CCP and this is considered correct. For this step the interpretation of the decision tree in Table 9 is not correct and the answer to Q2 is probably ‘No’ instead of ‘Yes’. The applicant recognises that microbiological and chemical hazards might be present resulting in an ‘off spec’ product. Assuming that ‘off spec’ means ‘material out of specification’, the sampling of the end product is listed as a preventive measure and the reprocessing of the material is applied when the samples are out of specifications. The applicant should clearly separate the storage step, which is not a CCP, and the sampling of the end product, which is a mean of verification of the efficacy of the process and of the HACCP plan.
All in all, the reception of AF and UCO must not be considered a CCP, but a prerequisite, while the other CCPs identified by the applicant, represented by the reactors and product strippers, are considered correct, with clear limits, means of verification and corrective actions. However, the mean of verification of the successful entry and exit of the materials in the stripper tower is unclear.
3.6. Risk associated with interdependent processes
3.6.1. Risk associated with interdependent processes as provided by the applicant
This section provides a description of the risks associated with the interdependent processes and of the mitigating procedures for dealing with these risks.
3.6.1.1. Pretreatment potential by‐products
Pretreatment processes are dependent on the quality of the sourced material. Should a material have had to undergo a pretreatment step before being acceptable for co‐processing at the site, those steps may include degumming, bleaching and deodorisation. Those potential steps would occur at another facility (third party) that has the capability to carry out those processes. Those facilities would be licensed and approved companies with permitted methods to carry out the necessary steps that would happen before any material arrives at bpRR.
The following description provides a high‐level explanation of the processes involved and identifies the by‐products of the potential processes used. Pretreatment steps may involve a front‐end polyethylene removal option. In this case, a pressure leaf filter would be producing cake periodically.
During degumming (phosphorous and metals removal), citric acid helps to create hydratable oil and produces a gum (phosphatide) stream. Gums are a potential by‐product and will possibly go into a fermentation process (fermenting the fatty matter and producing biogas that may be used for electricity production). The gums are a milky to gummy by‐product that is dependent on the renewable feedstock. It is organic and non‐hazardous and has a reuse potential in other industries such as fermentation and incineration. The by‐product can be dewatered to reduce volume, however whether it needs dewatering depends on the reuse/disposal option.
The disposal of the gums will be done as per a legislated and approved method by the pretreatment processing company.
If bleaching occurs, its purpose is to remove the colour and remaining contaminants. In this process, bleaching earth and filter aid are used and a filter cake is created. The filter cake would contain spent bleaching earth and oil residue. It is also considered pyrophoric and must be sent to specialised (authorised) landfills.
Once the material has been treated, it will be sent to bpRR and will be checked against the acceptance criteria.
3.6.1.2. Storage
All end products (renewable fuels created from the co‐processing process) are stored in specifically designated tanks in the bunded and monitored tank farm (this applies equally to the storage tanks that also contain LGO, AF and UCO, although these are separately designated tanks). All storage tanks must comply with Dutch environmental regulations (PGS‐29) that state how storage tanks must be designed, operated and maintained. Numerous processes and procedures have been developed and used to comply with these governmental standards.
Any environmental risk or risk to human or animal health due to spills or leaks during storage or filling is mitigated by the use of bunded spaces and sumps that can be pumped back to designated storage.
Additionally, during any maintenance or cleaning activities, processes and procedures are in place to avoid any contact with hydrocarbons (which may contain AF and UCO or renewable fuels). Due to the fact that other hazards (toxic gases, confined spaces, energy isolation or an oxygen‐deficient atmosphere) exist, further precautionary health and safety measures are in place for the avoidance of any incidents, including exposure to pathogens.
3.6.1.3. Transport
Similar to sourcing AF and UCO, the end products are sold through the trading department (BP Trading and Shipping) based in London. The business entity BP Oil International Limited will sell all renewable fuels and is a registered trader for these products as well.
The sale of the end product will be done in coordination with bpRR and BP Oil International Limited. Buyers (local or international) of the end product must also be licensed businesses with the infrastructure to operate facilities that can handle the quantities in question.
In order to deliver the end product to these companies, transportation of the end product will occur by one of two methods. Either the end product will be sent from bpRR via a pipeline to a storage, similar to the existing storage tanks located at bpRR, or it will be sent via a barge or tanker.
3.6.1.3.1. Barges and tankers
Barges and tankers (sea‐going vessels) that will transport the renewable fuels off site will be constructed from the appropriate materials and by licensed transporters. All vessels will be pre‐selected based on qualifications and suitability (as per bpRR standardised operational procedures and in conjunction with BP Oil International), labelled with the appropriate signage and all loads will be accompanied by the correct legal and environmental paperwork. They will comply with docking and loading procedures while moored at bpRR.
If a vessel containing renewable fuel crashed and, in the extreme scenario, was breached and the renewable fuel was discharged, exposure to pathogens from the renewable fuel would not be expected.
3.6.1.3.2. Pipelines
Pipelines that will transport the renewable fuels off site will be constructed from appropriate materials and sent off site with certified pumps. The destination of the renewable fuels through pipelines may vary in location. However, it will also require a storage tank before the renewable fuel is sold to an end customer for the purposes of combustion.
In the event that a pipeline leaked or was damaged, exposing materials to the environment, procedures for spill response are in place and exposure to pathogens from the renewable fuel would not be expected.
3.6.1.4. Safe disposal of by‐products
3.6.1.4.1. Wastewater generated in the hydrofiners
Wastewater is produced as a by‐product from the AF/UCO and LGO having gone through the process units. Only after having gone through the respective reactors, in which risk reduction has been achieved, will wastewater be created as a by‐product and therefore be free of any pathogens.
As stated in Section 3.4.1.3, using the extrapolation methodology, any spore‐forming bacteria would be reduced by a ■■■■■ (calculated by the difference in the log values between 270 and 90°C) and therefore is not expected to appear in any wastewater that is generated by the hydrofiners.
The wastewater is sent via sewers to the ETP for processing. Once the wastewater has been processed, it is discharged into the local harbour. The risk of exposure to any pathogens is expected to be low.
3.6.1.4.2. Gases generated in the hydrofiners
Gases are produced as a by‐product from the AF and UCO having gone through the reactors of the process units. Therefore, the level of risk reduction will also be of the ■■■■■ The gases will be free from pathogens and will be fed to the fuel gas system as fuel for combustion (incineration) into the furnaces for the purposes of heating hydrocarbons. Exposure to pathogens via this route is also not expected.
During the desulfurisation process, ammonia (NH3) is formed in small quantities. This ammonia is extremely soluble in water and, with a small amount of water injected into the process, the ammonia will dissolve. The injection of water occurs at the heat exchangers before the high‐pressure separators.
Hydrogen sulfide (H2S) is handled by the low‐pressure separators. When the pressure is released (lowered), the gases residing in the mixture (including H2S) are freed. These gases are sent to the H2S gas absorber where H2S is removed (scrubbed).
3.6.1.5. Planned or unplanned unit outages
In the event of an outage, whether it is planned or unplanned, a set of events takes place in which the flows inside the process units are redirected to storage tanks, called slops tanks. Slops tanks are a specific set of tanks designated to store any material that is meant for reprocessing. At this stage, the material is once again blended and sent to the process units.
Should this occur and the material that has not passed through the reactors is sent to the slops tank, in the second occurrence, it will pass through the process units and attain the proper level of risk reduction. This hazard is identified in Section 6.2 (the HACCP) in which the corrective action is for the material to be sent to storage for reprocessing.
As the process unit is contained, as well as all the piping, the risk of exposure of pathogens to the atmosphere is not expected. Re‐routing material to other storage tank does not involve any of the material being exposed to the open atmosphere.
3.6.1.5.1. Planned outage
In the event of a planned outage related to maintenance, because it would be a planned event, any UCO and AF feedstock would not be present before the shutdown. In such a case, bpRR will have stopped incorporating the feed into the hydrofiners before any shutdown processes commence. This negates any potential risk.
During any given maintenance that involves any part of the process units to be dismantled or opened, bpRR follows rigorous safety reviews and procedures before commencing the work [all of which fall under bp's OMS and Rotterdam OMS (ROMS) procedures].
3.6.1.5.2. Unplanned outage
In the event of an unplanned outage, in which the processing units unexpectedly stop operating and UCO and AF could be present, the hydrofiners would enter a re‐circulation mode that would prevent any further product run down to enter the tanks. In such a case, the product will become off spec, as it will not have been exposed to all the necessary environments. Some of this unfinished product will then be routed to the slops tanks and later be reprocessed. This portion of the product is at the end of the process (post‐stripper). However, most of it will remain within the hydrofiners and stay in re‐circulation mode until the product run down is deemed once again to meet the specification criteria of the final products.
There are several aspects to note in this situation. The first is that material that has been processed beyond the reactors will have already undergone a sufficient risk reduction and should pose no further threat. The second aspect is that any material residing in the sections before the reactors, will continue to stay in a re‐circulation mode until the unit is fully operational again and then proceed into the reactor at the correct operational temperature and pressure. This operational method also prevents any exposure to the feedstocks and allows the risk reduction to be achieved.
Last, should an unplanned outage occur in which mechanical procedures (maintenance involving physical repairs in which the possibility of exposure of hydrocarbons, UCO and AF exists) are conducted, all feedstocks would be drained via connections (hoses) and the feedstock would be transported in vacuum trucks to the appropriate tanks. This would prevent any exposure to the environment and surrounding population. Staff carrying out these activities would be qualified and following existing procedures on how to conduct such activities.
3.6.1.6. Corrective actions
The corrective actions that bpRR's staff are required to carry out in the event that the operations fall outside of the necessary conditions are based on a variety of scenarios that could happen. As it is bpRR's priority to ensure safe operations, numerous philosophies, systems, processes, procedures, guides, trainings and certifications have been incorporated into our daily ways of working to ensure all preventive measures are taken towards safety and the environment.
In that regard, BP (Global) developed an OMS and bpRR has developed the ROMS, which is a set of documents that addresses all necessary aspects and practices that are defined in the OMS system.
In any and every case for which the critical control points are not being met, corrective procedures are in place. For all process‐related equipment, the operating conditions are monitored through the DCS system. When there is an alarm, the board operator will react. For the hydrofiners, the process will then be suspended. An investigation will commence to understand the root cause of the alarm and all necessary actions will be carried out to rectify the situation. Once the situation has been addressed (including repairs, replacements, etc.), the process will be restarted and the cycle will be repeated.
3.6.2. BIOHAZ Panel assessment of the risk associated with interdependent processes
The applicant provided a detailed description of the interdependent processes related to transport and storage and the procedures that would be implemented in dealing with risks. It is not specifically mentioned whether procedures are undertaken in compliance with the requirements set out in Article 21 of Regulation (EC) No 1069/2009, Article 17 and Annex VIII of Commission Regulation (EU) No 142/2011 and various other parts of these regulations. However, the measures in place should provide a high level of protection against biological and other hazards.
The applicant provided detailed information on the processes (degumming, bleaching and deodorisation) involved in the production of by‐products during pretreatment. Information was provided on the procedure for disposing the by‐products of the bleaching process. The applicant stated that gums from the degumming process are disposed of in accordance with the legislation, but information was not provided on what legislation is applicable in this case and the precise method of disposal that is used.
The applicant also provided information on the by‐products produced during the core steps of the alternative method. During the reactor step (desulfurisation, denitrification, olefin saturation, and aromatic saturation), various by‐products including propane (C3H8), carbon monoxide (CO), carbon dioxide (CO2), hydrogen sulfide (H2S) and ammonia (NH3) are produced. The applicant described the process for dealing with these by‐products. For example, the ammonia is dissolved in water and the H2S is sent to the H2S gas absorber and removed by scrubbing. All of these by‐products are exposed to the high temperatures associated with the alternative process, ensuring that they should be free of any pathogens. Similarly, wastewater will only be created as a by‐product after it has gone through the process units. This should ensure that it will also be free of any pathogens. There is a comprehensive process in place for dealing with wastewater generated in the plant.
Overall, the information provided by the applicant indicates that comprehensive and adequate procedures are in place for dealing with any risks associated with interdependent processes.
3.7. Risk associated with the intended end use of the product
3.7.1. Risk associated with the intended end use of the product as provided by the applicant
The end product of the proposed method is renewable fuels used for transportation. Considering the nature and planned uses of this final product, exposure of animals or humans to pathogens from the interdependent processes and the intended end use of the product is not expected.
The renewable fuel will be blended with fossil fuel for use in domestic and commercial vehicles and will be dispensed at retail filling stations (or other equivalent authorised dispensing stations). A limited number of potential routes of infection of humans with pathogens exist because of the residual risk associated with the renewable fuels that are blended with fossil fuels.
There are two main routes of potential infection that exist: oral and subcutaneous. It is highly unlikely that renewable fuels would be intentionally ingested or inhaled. The subcutaneous route represents a more viable route of exposure, for instance through a wound or a laceration. In the situation of an open wound or laceration, the quantity of renewable fuel that could penetrate the underlying cells is estimated as less than a millilitre. Therefore, the exposure to pathogens via a filling vehicle is not expected.
The possibility of exposure of an animal either by ingesting or being exposed by the subcutaneous route to pathogens in renewable fuel blended with fossil fuel is not expected. For this to occur, it would be necessary for either a spillage or other major incident to happen. All areas of the storage facilities are bunded to an appropriate capacity. The processing facilities have sumps that can be pumped back into a storage tank. As a result of the bunds and sumps, it is extremely unlikely that any animal would come into contact with 100% renewable fuel. Similar environmental protection strategies and on‐site security would exist at other facilities. Additionally, due to the hazardous nature of the fuel, retail filling stations are extremely unlikely to have exposed fuel present. Therefore, the exposure of an animal to pathogens via contact with sufficient renewable fuels is not expected.
3.7.2. BIOHAZ Panel assessment of the risk associated with the intended end use of the product
The end product of the proposed alternative method are renewable fuels. Considering their nature and uses, no additional risks associated with the intended end use of the product are planned. Indeed, the safety measures applied during their transport and sale will avoid exposure to hazards of humans and animals during normal operation.
4. Conclusions
The raw materials to be processed by the proposed alternative method for the production of renewable fuels are rendered AF and UCO, derived from Category 3 ABPs. AF would be pretreated using methods 1–5 or method 7, whereas UCO would not be processed using any of these methods.
The alternative method consists of a catalytic co‐processing hydrotreatment using a middle distillate such as LGO, followed by a stripping step. The materials must be submitted to a pressure of at least 60 bars at a temperature and time of at least 270°C for at least 4.7 min.
The EFSA BIOHAZ Panel considered that a reduction of 5 log10 and 3 log10 of the relevant pathogenic bacteria and thermoresistant viruses, respectively, as identified in the hazard identification, should be demonstrated to validate the alternative method. If spore‐forming pathogenic bacteria are considered relevant in the hazard identification, the required level of inactivation shall be a 5 log10 reduction of spores from pathogenic bacteria, with the exception of spores of C. botulinum for which a 12 log10 reduction will be required, as for processing canned petfood. Alternatively, spores of pathogenic bacteria can be directly used as a primary target, given their high level of resistance to heat. For both spore‐forming and non‐spore‐forming bacteria and viruses, adequate alternative non‐pathogenic indicator or surrogate organisms, with at least the same level of resistance, may be also considered for demonstrating an equivalent level of reduction in the substrate of interest.
The conclusions of the assessment are:
The applicant did not perform a full hazard identification detailing all the relevant biological hazards for human and animal health, related to the origin and category of the material to be processed. However, the approach followed by the applicant is consistent with one of the possible scenarios considered acceptable: the selection of spores from non‐pathogenic spore‐forming indicator bacterial species (in this case, B. subtilis and D. kuznetsovii) as a primary target to demonstrate a sufficient level of hazard reduction, considering that any process achieving a significant level of reduction of them will ensure at least a similar level of reduction of all biological hazards possibly present in the Category 3 material.
Despite the limitations highlighted in the assessment of the extrapolation analyses performed by the applicant, the dossier and additional literature contain sufficient evidence to support that the proposed alternative method can achieve a sufficient level of hazard reduction (e.g. a reduction of at least 5 log10 of B. subtilis and 12 log10 of C. botulinum).
In the HACCP plan, the reactors and product strippers were identified by the applicant as CCPs and this was considered correct. The critical limits, means of monitoring and verification, and corrective actions associated with the CCPs were clear except for the means of verification of the successful entry and exit of the materials in the stripper tower. The applicant identified the acceptance of the material on site as a CCP, whereas this should be a prerequisite.
The information provided by the applicant suggests that comprehensive and adequate procedures are in place for dealing with any risks associated with interdependent processes and the end use of the product.
Overall, the alternative method under assessment can be considered to be at least equivalent to the processing methods currently approved in the Commission Regulation (EU) No 2011/142.
5. Documentation provided to EFSA
October 2021:
Letter. Re: Application for alternative processing method for animal by‐products. Plant Supply Chain and Food Quality Department, Ministry of Agriculture, Nature and Food Quality. 22 October 2021.
Annex I: Application of the company bp Raffinaderij Rotterdam B.V. Application for alternative method for the processing of Animal by‐products at bp Rotterdam Refinery
Appendix 1 – Specifications criteria (included in Annex I).
Appendix 2 – Recycled Cooking Oils: Assessment of risks for public. European Parliament Directorate‐General for Research Directorate A. The STOA Programme. September 2000 (separate document).
Appendix 3 – Effect of lipid materials on heat resistance of bacterial spores (separate document). Molin N and Snygg BG, 1967. Effect of lipid materials on heat resistance of bacterial spores. Applied Microbiology, 15(6), 1422–1426. https://doi.org/10.1128/am.15.6.1422-1426.1967
Appendix 4 – Heat resistance of Bacillus spores (separate document). Berendsen EM, 2016. Heat resistance of Bacillus spores: natural variation and genomic adaptation. PhD Thesis, University of Groningen, Rijksuniversiteit Groningen.
Appendix 5 – Effects of high hydrostatic pressure on Clostridium sporogenes (separate document). Mills G, Earnshaw R and Patterson MF, 1998. Effects of high hydrostatic pressure on Clostridium sporogenes spores. Letters in Applied Microbiology, 26(3), 227–230. https://doi.org/10.1046/j.1472-765x.1998.00329.x
Appendix 6 – Inactivation of Clostridium botulinum type A spores by high‐pressure processing at elevated temperatures (separate document). Reddy NR, Solomon HM, Tetzloff RC and Rhodehamel EJ, 2003. Inactivation of Clostridium botulinum type A spores by high‐pressure processing at elevated temperatures. Journal of Food Protection, 66(8), 1402–1407. https://doi.org/10.4315/0362-028x-66.8.1402
Annex II: Assessment of the application of a new alternative processing method by bp Raffinaderij Rotterdam B.V. Netherlands Food and Consumer Product Safety Authority. Ministry of Agriculture, Nature and Food Quality. 19 June 2021.
Annex III: Contact address.
January 2022
Annex I: Application of the company bp Raffinaderij Rotterdam B.V. Application for alternative method for the processing of animal by‐products at bp Rotterdam Refinery. Amended
Annex I: Application of the company bp Raffinaderij Rotterdam B.V. Application for alternative method for the processing of animal by‐products at bp Rotterdam Refinery. With confidentiality claim.
March 2022
Annex I: Application of the company bp Raffinaderij Rotterdam B.V. Application for alternative method for the processing of animal by‐products at bp Rotterdam Refinery. With confidentiality claim. Amended.
May 2022
Annex I: Application of the company bp Raffinaderij Rotterdam B.V. Application for alternative method for the processing of animal by‐products at bp Rotterdam Refinery. With confidentiality claim amended.
July 2022
Annex I: Application of the company bp Raffinaderij Rotterdam B.V. Application for alternative method for the processing of animal by‐products at bp Rotterdam Refinery. With confidentiality claim amended.
Appendix 7 – Control of bacterial spores
Appendix 8 – Thermophilic methanol utilisation by sulfate‐reducing bacteria
Appendix 9 – Thermophilic anaerobic spore formers
Abbreviations
- ABP
animal by‐products
- ADO
automotive diesel oil
- AF
animal fats
- AHAW
Animal Health and Welfare
- BAT
best available techniques
- BIOHAZ
Biological Hazards
- CA
Competent Authority
- CCP
critical control point
- CDU
crude distillation units
- DCS
distributed control system
- ETP
effluent treatment plant
- HACCP
Hazard Analysis and Critical Control Points
- LGO
light gas oil
- LPG
liquid propane gas
- OMS
Operating Management System
- PATP
pressure‐assisted thermal processing
- ROMS
Rotterdam Operating Management System
- TSE
transmissible spongiform encephalopathies
- UCO
used cooked oils
- WG
Working Group
Appendix A – Process flow diagram of the hydrofiners
Figure A.1.
The process flow of hydrofiner 3 and hydrofiner 1
Appendix B – Decision tree to identify critical control points (CCP)
Hazard Analysis and Critical Control Point (HACCP) System and Guidelines for its Application. Annex to CAC/RCP 1–1969, Rev. 3 (1997).
Suggested citation: EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) , Koutsoumanis K, Allende A, Bolton D, Bover‐Cid S, Chemaly M, Davies R, De Cesare A, Herman L, Hilbert F, Lindqvist R, Nauta M, Peixe L, Ru G, Simmons M, Skandamis P, Suffredini E, Fernández Escámez P, Griffin J, Ortiz‐Pelaez A and Alvarez‐Ordoñez A, 2022. Scientific Opinion on the evaluation of a multi‐step catalytic co‐processing hydrotreatment for the production of renewable fuels using Category 3 animal fat and used cooking oils. EFSA Journal 2022;20(11):7591, 42 pp. 10.2903/j.efsa.2022.7591
Requestor European Commission
Question number EFSA‐Q‐2021‐00625
Panel members Ana Allende, Avelino Alvarez‐Ordoñez, Declan Bolton, Sara Bover‐Cid, Marianne Chemaly, Robert Davies, Alessandra De Cesare, Lieve Herman, Friederike Hilbert, Konstantinos Koutsoumanis, Roland Lindqvist, Maarten Nauta, Luisa Peixe, Giuseppe Ru, Marion Simmons, Panagiotis Skandamis and Elisabetta Suffredini.
Declarations of interest If you wish to access the declaration of interests of any expert contributing to an EFSA scientific assessment, please contact interestmanagement@efsa.europa.eu.
Acknowledgements The BIOHAZ Panel wishes to thank Maria Francesca Iulietto for the support provided to this scientific output.
The scientific output published implements EFSA's decision on the confidentiality requests submitted on specific items. As certain items have been awarded confidential status by EFSA they are consequently withheld from public disclosure by redaction.
Adopted: 28 September 2022
Notes
Regulation (EC) No 1069/2009 of the European Parliament and of the Council of 21 October 2009 laying down health rules as regards animal by‐products and derived products not intended for human consumption and repealing Regulation (EC) No 1774/2002 (Animal by‐products Regulation). OJ L 300, 14.11.2009, pp. 1–33.
Commission Regulation (EU) No 142/2011 of 25 February 2011 implementing Regulation (EC) No 1069/2009 of the European Parliament and of the Council laying down health rules as regards animal by‐products and derived products not intended for human consumption and implementing Council Directive 97/78/EC as regards certain samples and items exempt from veterinary checks at the border under that Directive. OJ L 54, 26.2.2011, pp. 1–254.
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. OJ L 31, 1.2.2002, pp. 1–24.
Commission Regulation (EU) No 749/2011 of 29 July 2011 amending Regulation (EU) No 142/2011 implementing Regulation (EC) No 1069/2009 of the European Parliament and of the Council laying down health rules as regards animal by‐products and derived products not intended for human consumption and implementing Council Directive 97/78/EC as regards certain samples and items exempt from veterinary checks at the border under that Directive. OJ L 198, 30.7.2011, pp. 3–22.
Level of risk reduction is used interchangeably in the opinion with level of hazard reduction.
Regulation (EC) No 1774/2002 of the European Parliament and of the Council of 3 October 2002 laying down health rules concerning animal by‐products not intended for human consumption OJ L 273, 10.10.2002, p. 1.
According to Commission Regulation (EU) 142/2011, Annex XIII, Chapter II, point 3 ‘canned petfood must be subjected to heat treatment to a minimum Fc value of 3’.
European Commission Food Animal By products: Approved Establishments, https://ec.europa.eu/food/safety/animal-by-products/approved-establishments_en (Accessed 2020‐10‐9).
The data point provided by the applicant in the dossier is not correct. It should be 340 min.
References
- Berendsen E, 2016. Heat resistance of Bacillus spores: natural variation and genomic adaptation. PhD Thesis, University of Groningen, Rijksuniversiteit Groningen, The Netherlands.
- Berendsen EM, Zwietering MH, Kuipers OP and Wells‐Bennik MHJ, 2015. Two distinct groups within the Bacillus subtilis group display significantly different spore heat resistance properties. Food Microbiology, 45(Pt A), 18–25. 10.1016/j.fm.2014.04.009 [DOI] [PubMed] [Google Scholar]
- Black EP, Setlow P, Hocking AD, Stewart CM, Kelly AL and Hoover DG, 2007. Response of spores to high‐pressure processing. Comprehensive Reviews in Food Science and Food Safety, 6, 103–119. 10.1111/j.1541-4337.2007.00021.x [DOI] [Google Scholar]
- Brown KL, 2000. Control of bacterial spores. British Medical Bulletin, 56(1), 158–171. 10.1258/0007142001902860 [DOI] [PubMed] [Google Scholar]
- Diao MM, André S and Membré JM, 2014. Meta‐analysis of D‐values of proteolytic Clostridium botulinum and its surrogate strain Clostridium sporogenes PA 3679. International Journal of Food Microbiology, 174, 23–30. 10.1016/j.ijfoodmicro.2013.12.029 [DOI] [PubMed] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) , 2004. Scientific Opinion on the safety Vis‐à‐Vis biological risk including TSEs of the application on pastureland of organic fertilisers and soil improvers. EFSA Journal 2004;2(3):40, 10 pp. 10.2903/j.efsa.2004.40 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) , 2005. Scientific Opinion on the safety vis‐à‐vis biological risks of biogas and compost treatment standards of animal by‐products (ABP). EFSA Journal 2005;3(9):264, 21 pp. 10.2903/j.efsa.2005.264 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) , 2007. Scientific Opinion on a request from the European Commission on the safety vis‐à‐vis biological risk of the mesophilic process of biogas and compost treatment of animal by‐products (ABPs). EFSA Journal 2007;5(3):465, 16 pp. 10.2903/j.efsa.2007.465 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) , 2009. Statement on technical assistance related to the EFSA opinion on transformation of animal by‐products into biogas and compost. EFSA Journal 2009;7(11):1370, 8 pp. 10.2903/j.efsa.2009.1370 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) , 2010. Statement on technical assistance on the format for applications for new alternative methods for animal by‐products. EFSA Journal 2010;8(7):1680, 12 pp. 10.2903/j.efsa.2010.1680 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) , 2015a. Scientific Opinion on a continuous multiple‐step catalytic hydrotreatment for the processing of rendered animal fat (Category 1). EFSA Journal 2015;13(11):4307, 23 pp. 10.2903/j.efsa.2015.4307 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) , 2015b. Scientific Opinion on an alternative method for the hygienic treatment of bovine colostrum through a series of filtration steps. EFSA Journal 2015;13(6):4319, 19 pp. 10.2903/j.efsa.2015.4139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) , 2015c. Risk to public and/or animal health of the treatment of dead‐inshell chicks (Category 2 material) to be used as raw material for the production of biogas or compost with Category 3 approved method. EFSA Journal 2015;13(11):4306, 46 pp. 10.2903/j.efsa.2015.4306 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) , 2017. Scientific Opinion on the evaluation of the Application for new alternative biodiesel production process for rendered fat of Cat 1 (BDI‐RepCat process, AT). EFSA Journal 2017;15(11):5053, 22 pp. 10.2903/j.efsa.2017.5053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) , 2018. Scientific Opinion on the evaluation of the application for a new alternative processing method for animal by‐products of Category 3 material (ChainCraft B.V.). EFSA Journal 2018;16(6):5281, 23 pp. 10.2903/j.efsa.2018.5281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) , 2020a. Evaluation of an alternative method for production of biodiesel from processed fats derived from Category 1, 2 and 3 animal by‐products (submitted by College Proteins). EFSA Journal 2020;18(4) e06089, 29 pp. 10.2903/j.efsa.2020.6089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) , 2020b. Scientific Opinion on the evaluation of alternative methods of tunnel composting (submitted by the European Composting Network). EFSA Journal 2020;18(8):6226, 36 pp. 10.2903/j.efsa.2020.6226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) , Koutsoumanis K, Allende A, Alvarez‐Ordonez A, Bolton D, Bover‐Cid S, Chemaly M, Davies R, De Cesare A, Hilbert F, Lindqvist R, Nauta M, Peixe L, Ru G, Simmons M, Skandamis P, Suffredini E, Cocconcelli PS, Fernandez Escamez PS, Prieto‐Maradona M, Querol A, Sijtsma L, Evaristo Suarez J, Sundh I, Vlak J, Barizzone F, Hempen M and Herman L, 2022. Statement on the update of the list of QPS‐recommended biologicalagents intentionally added to food or feed as notified to EFSA 15: suitability of taxonomic units notifiedto EFSA until September 2021. EFSA Journal 2022;20(1):7045, 40 pp. 10.2903/j.efsa.2022.7045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) and EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) , 2011. Scientific Opinion on hatchery waste as animal by‐products. EFSA Journal 2011;9(7):2321, 35 pp. 10.2903/j.efsa.2011.2321 [DOI] [Google Scholar]
- Enache E and Podolak R, 2013. Chapter 27. Thermophilic anaerobic sporeformers. In: Y Salfinger and MR Tortorello (eds.). Compendium of Methods for the Microbiological Examination of Foods. APHA Press. 10.2105/MBEF.0222.032 [DOI] [Google Scholar]
- Goorissen HP, 2002. Thermophilic methanol utilization by sulfate reducing bacteria. PhD Thesis, University of Groningen, Rijksuniversiteit Groningen, The Netherlands.
- Lima LJR, Kamphuis HJ, Nout MJR and Zwietering MH, 2011. Microbiota of cocoa powder with particular reference to aerobic thermoresistant spore‐formers. Food Microbiology, 28(3), 573–582. 10.1016/j.fm.2010.11.011 [DOI] [PubMed] [Google Scholar]
- Masana MO and Baranyi J, 2000. Adding new factors to predictive models: the effect on the risk of extrapolation. Food Microbiology, 17(4), 367–374. 10.1006/fmic.1999.0326 [DOI] [Google Scholar]
- Mills G, Earnshaw R and Patterson MF, 1998. Effects of high hydrostatic pressure on Clostridium sporogenes spores. The Society of Applied Microbiology, 26, 277–230. 10.1046/j.1472-765x.1998.00329.x [DOI] [PubMed] [Google Scholar]
- Molin N and Snygg BG, 1967. Effect of lipid materials on heat resistance of bacterial spores. Applied Microbiology, 15(6), 1422–1426. 10.1128/am.15.6.1422-1426.1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peleg M, 2021. The thermal death time concept and its implications revisited. Food Engineering Reviews, 13(2), 291–303. 10.1007/s12393-021-09279-8 [DOI] [Google Scholar]
- Ramirez‐Lopez L, 2006. Heat inactivation of thermo‐resistant bacteria isolated from poultry offal. MS Thesis, Clemson University, USA. Available online: https://tigerprints.clemson.edu/all_theses/17 [Accessed: 2 November 2021].
- Reddy NR, Solomon HM, Tetzloff RC and Rhodehamel EJ, 2003. Inactivation of Clostridium botulinum Type A spores by high‐pressure processing at elevated temperatures. Journal of Food Protection, 66(8), 1402–1407. 10.4315/0362-028x-66.8.1402 [DOI] [PubMed] [Google Scholar]
- Van Asselt ED and Zwietering MH, 2006. A systematic approach to determine global thermal inactivation parameters for various food pathogens. International journal of food microbiology, 107, 73–82. [DOI] [PubMed] [Google Scholar]
- Wells‐Bennik MHJ, Eijlander RT, den Besten HMW, Berendsen EM, Warda AK, Krawczyk AO, Nierop Groot MN, Xiao Y, Zwietering MH, Kuipers OP and Abee T, 2016. Bacterial spores in food: Survival, emergence, and outgrowth. Annual Review of Food Science and Technology, 7, 457–482. 10.1146/annurev-food-041715-033144 [DOI] [PubMed] [Google Scholar]
- Wijnands LM, van der Meij‐Florijn A, Delfgou‐van Asch EHM and van Leusden FM, 2009. Heat sensitivity of Clostridium perfringens . RIVM Report 330371004/2009, Bilthoven, The Netherlands. Available online: https://www.rivm.nl/bibliotheek/rapporten/330371004.pdf [Accessed: 2 November 2021].


