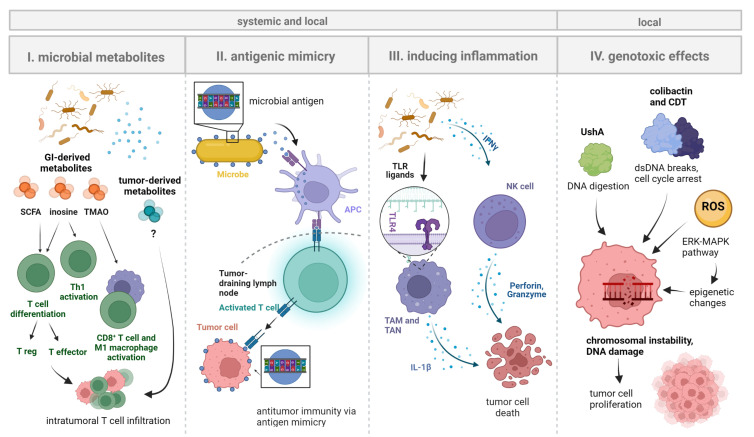
Figure 2.
Systemic and local effects of microbes on tumorigenesis and immune cells. Shown are principal mechanisms by which microbiota may influence tumorigenesis and local immune cells that take effect on cancer immunotherapy efficacy. Microbiota can have direct effects on the immunotherapy target cells (ie, cancer cells) or regulate the amplitude and quality of the anticancer immune response both on and off treatment: (1) Microbial metabolites (of GI microbiota, likely also of local microbiota) may promote T cell activation and differentiation, resulting in the accumulation of both proinflammatory (eg, effector T cells) and immunosuppressive (eg, T regulatory cells) immune cell populations in the TME. (2) MHC class II-expressing cells (eg, DCs) presenting microbial antigens may activate T cells in tumor-draining lymph nodes. Molecular mimicry of tumor antigens by microbial peptides would result in tumor-directed T cell activity, thereby influencing cancer immunotherapy efficacy. (3) Bacterial molecules (pathogen-associated molecular patterns) such as LPS may bind TLRs (eg, TLR4) on TAMs/TANs or directly activate tumor-associated NK cells (eg, via IFNγ signaling). This may activate downstream pro-inflammatory pathways and/or promote tumor cell death. (4) Genotoxic microbial products can induce DNA damage in cancer cells to reshape the tumor genome and promote cancer cell proliferation. Examples include colibactin and CDT that both induce transient cell cycle arrest (promoting the occurrence of genomic errors during the repair process) and double-strand breaks leading to chromosomal instability139 140as well as the novel genotoxin ushA (acting via DNA digestion) and ROS that directly damage cancer cells and/or induce epigenetic changes via ERK-MAPK signaling. Figure is created with BioRender.com.73 122 (IMPORTANT: move this citation to the sentence before right after "ERK-MAPK signaling".) APC, antigen-presenting cell; CDT, cytolethal distending toxin; DC, dendritic cell; LPS, lipopolysaccharide, NK, natural killer (cell); ROS, reactive oxygen species; SCFA, short-chain fatty acid; TLR, Toll-like receptor; TAM, tumor-associated macrophage; TAN, tumor-associated neutrophil; TMAO, trimethylamine N-oxide; TME, tumor microenvironment.