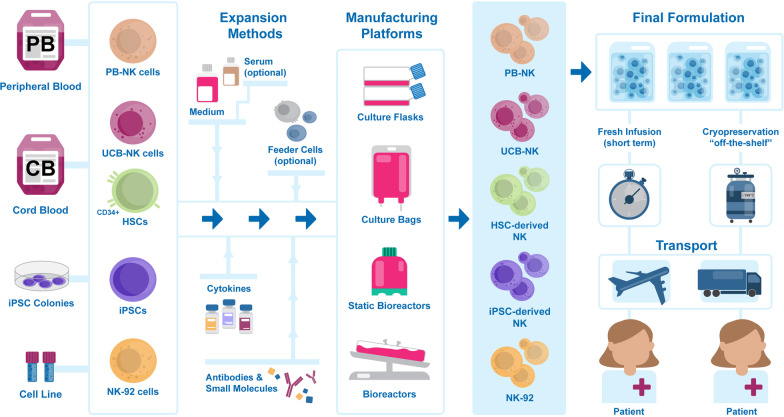
Fig. 3.
Natural killer cell therapy manufacturing process pipeline. From left to right: NK cells are isolated from peripheral blood (PB-NK), from umbilical cord blood (UCB-NK), derived from cord blood CD34-positive hematopoietic stem cells (HSCs), from induced hematopoietic stem cells (iPSCs), or from in vitro propagated cell lines (NK-92). Cell expansion (and differentiation) is achieved ex vivo by culture in suitable medium, optionally supplemented with serum, or in presence of feeder cells. Cytokines, antibodies, and other small molecules are added to support cell expansion and maturation. Manufacturing platforms include cell culture flasks, bags, and static and dynamic bioreactors. Once the appropriate number of cells with the desired phenotype is obtained, NK cells are harvested and collected in the final formulation before infusion into patients. Fresh products must be transported to the patient and administered shortly after collection. Cryopreserved products can be stored in appropriate conditions and be delivered to the patient as needed, for on-site thawing and true “off-the-shelf” administration