Abstract
Background:
Postnatal maternal anxiety is common (estimates as high as 40% prevalence), and is associated with altered mother-infant interactions (e.g., reduced maternal emotional expression and engagement). Neural circuitry supporting infants’ face- and emotion-processing develops in their first year. Thus, early exposure to maternal anxiety may impact infants’ developing understanding of emotional displays. We examine whether maternal anxiety is associated with individual differences in typically developing infants’ neural responses to emotional faces.
Methods:
142 mother-infant dyads were assessed when infants were 5-, 7-, or 12-months-old. Infants’ electroencephalographic (EEG) data were recorded while passively viewing female happy, fearful, and angry faces. Three event-related potential (ERP) components, each linked to face- and emotion-processing, were evaluated: NC, N290, and P400. Infant ERP amplitude was related to concurrent maternal-report anxiety assessed with the Spielberger State-Trait Anxiety Inventory (Trait form).
Results:
Greater maternal anxiety predicted more negative NC amplitude for happy and fearful faces in left- and mid-central scalp regions, beyond covarying influences of maternal depression symptoms, infant negative emotionality, and infant age.
Conclusions:
Perinatal maternal anxiety is related to infants’ neural processing of emotional expressions. Infants of mothers endorsing high trait anxiety may need additional attentional resources to process happy and fearful faces (expressions less likely experienced in mother-infant interactions). Future research should investigate mechanisms underlying this association, given possibilities include experiential, genetic, and prenatal factors.
Keywords: maternal anxiety, emotions, faces, EEG/ERP, Infants, NC, N290, P400
Infants are attuned to others’ faces early in development, and social signals conveyed through emotional expressions are a critical form of communication over the first year of life (Bayet & Nelson, 2019). Neural circuits supporting abilities to recognize, discriminate, and allocate attention to faces and emotional expressions (Xie & Richards, 2016; Jessen & Grossmann, 2015; Conte et al., 2020) have been identified, and clear neural responses to happy, fearful, and angry faces are evident soon after birth, as measured with event-related potentials (ERP) captured in the electroencephalogram (EEG) (Xie et al., 2019).
Considerably less is known about individual differences in the neural correlates of infants’ face- and emotion-processing. Understanding these individual differences is important given their associations with later emerging features of cognition and behavior (Dennis, Malone & Chen, 2009), personality/disposition (Martinos, Matheson, & de Haan, 2012), and disorders of social communication (O’Toole et al., 2013). For example, children with enhanced neural responses (greater N170 ERP amplitude) to angry faces are at greater risk for heightened anxiety assessed years later (O’Toole, DeCicco, Bertho, & Dennis, 2013). Such findings suggest that individual differences in early organization of face and emotion neural circuitry could be an important predictor of later social cognition and mental health, and point to possible underlying mechanisms supporting social-emotional development. Thus, understanding the factors associated with individual differences in infants’ neural processing of emotional cues offers a more complete understanding of the development of infants’ social perception and cognition, and risk for later cognitive and socioemotional difficulties. Indeed, uncovering specific genetic and environmental factors that shape infants’ developing face and emotion neural circuitry will elucidate more detailed pathways to children’s emerging socio-emotional strengths and challenges. Such work has implications for treatment, intervention, and prevention of socio-emotional clinical outcomes such as internalizing problems.
We investigate whether typically developing infants’ neural responses to emotional faces are associated with maternal anxiety assessed postnatally. Maternal anxiety is common in the perinatal period (prevalence rates from 15 to 40%; Dennis et al., 2017; Field, 2017). The neural circuitry supporting face- and emotion-processing develops during infants’ first year (Conte et al., 2020; Xie et al., 2019). Thus, exposure to maternal affect and behavior during this critical time may impact infants’ developing understanding of emotional displays. Notably, research suggests that maternal anxiety influences infants’ emotional environment. Anxious mothers display more negative, intrusive, and hostile behaviors (Crugnola et al., 2016), greater disengagement/withdrawal (Murray et al., 2007; Crugnola et al., 2016), reduced emotional tone (Nicol-Harper, Harvey, & Stein, 2007), lower sensitivity (Nicol-Harper et al., 2007; Warren et al., 2003), and more poorly coordinated emotional interactions with their infants (Beebe et al., 2010; Crugnola et al., 2016). Thus, compared to infants of non-anxious mothers, infants of anxious mothers have different affective and perceptual experiences, which may shape neural activation patterns underlying how infants process and interpret social/emotional signals.
Behavioral findings confirm a relation between maternal anxiety and infants’ emotion-processing. Compared to infants of mothers with low anxiety, infants of high-anxious mothers spend less time looking at fearful faces (Creswell et al., 2008), display greater irritability (Jover et al., 2014), show increased negativity (i.e., anger, annoyance, minimal engagement) during mother-infant interactions (Crugnola et al., 2016), show reduced social engagement (Feldman et al., 2009), and exhibit behavioral/emotion problems in later development (O’Connor, Hegron, Goldin, Beveridge, & Glover, 2002; Barker et al., 2011).
The present study examines the association between postnatal maternal anxiety and infant neural responses to emotional faces. Infants’ neural responses to emotional faces—if associated with maternal anxiety—could constitute an important marker for the impact of maternal anxiety, potentially revealing neural mechanisms by which maternal anxiety shapes infants’ social-emotional processing. The present study does not directly test these possibilities, but offers an important step in this valuable direction of research. Specifically, we test the hypothesis that postnatal maternal anxiety is associated with infants’ neural responses to happy, angry, and fearful faces in a large sample of typically developing infants ages 5-, 7-, and 12-months-old; critical points in the development of infant face- and emotion-processing neural circuitry (Leppanen & Nelson, 2009).
Two prior studies show associations among postnatal maternal psychological characteristics, caregiving behaviors, and 7-month-old typically developing infants’ neural responses to emotional expressions as assessed with EEG (de Haan et al., 2004; Taylor-Colls & Pasco Fearon, 2015). Both studies focused on the Negative Central (NC) ERP component—a negative deflection in the ERP waveform that occurs approximately 300–700 ms after viewing an event of interest (e.g., an emotional facial expression). The NC has been linked consistently to emotion-processing, and greater NC amplitude is thought to index increased attentional processes related to salience, significance, and/or familiarity of the emotional stimulus (de Haan, Johnson, & Halit, 2003). Taylor-Colls and Pasco Fearon (2015) found that 7-month-old infants of mothers rated as more sensitive (i.e., non-intrusive, non-hostile, more structured) during infant-child interactions had larger NC amplitudes to happy (relative to neutral) faces (computed as a happy-neutral difference wave), whereas NC amplitude to fearful (relative to neutral) faces was not related to maternal sensitivity. de Haan and colleagues (2004) found that infants whose mothers described their own and their infants’ personality/temperament as positive had smaller NC amplitudes to happy faces (raw amplitudes compared to fearful faces). Further, when infants of all temperament types were considered, those with more highly positive mothers had larger NCs to fearful faces (compared to raw happy NC amplitudes). Thus, a clear pattern of how maternal emotional traits relate to infants’ neural responses to emotional faces did not emerge from these two studies. However, together, they suggest that maternal characteristics are associated with infants’ underlying neural circuitry supporting face- and emotion-processing.
Perhaps most relevant to the present study, Otte et al. (2015) found that prenatal maternal anxiety was associated with 9-month-old infants’ neural responses (indexed via P350 auditory ERP) to multimodal emotional stimuli of vocalizations paired with emotional facial expressions (e.g., fearful vocalization paired with fearful face). These results are in line with neuroimaging research demonstrating associations between prenatal maternal anxiety and variation in newborn infants’ neural structures implicated in emotion-processing (e.g., insula) (Rifkin-Graboi et al., 2015). Most broadly, this research demonstrates that maternal anxiety—at least as assessed prenatally—may influence infants’ emotion-processing neural circuitry.
The present study sought to build on this prior foundational research. First, our focus on postnatal maternal anxiety. This focus provides a comparison to the positive maternal characteristics examined in de Haan et al. (2004) and Taylor-Colls & Pasco Fearon (2015). Further, it opens the possibility that aspects of infants’ postnatal experiences, which may vary relative to maternal characteristics, could influence infants’ developing emotion-processing neural circuitry in the first year of life (in contrast to the prenatal maternal anxiety focus in Otte et al., 2015, Rifkin-Graboi et al., 2015). Additionally, our use of single modal stimuli (faces only, versus vocalizations paired with faces in Otte et al., 2015) enables a more focused assessment of how visual processing of facial expressions may be altered by exposure to maternal anxiety. Moreover, to rigorously test whether maternal anxiety may uniquely contribute to individual differences in infants’ neural responses to emotional faces, we considered maternal depression symptoms (Gustafsson et al., 2018), infant negative emotionality (Henrichs et al., 2009), and family socioeconomic status (SES; Tomalski et al., 2013; Leach et al., 2017) as covariates, given that these variables can covary with both maternal anxiety and infant brain activity.
Second, we assessed infants at ages 5, 7, and 12 months to index emotion-processing neural circuitry at both an early point in development when neural specialization for faces and emotions is relatively premature, and at a later point after much specialization and maturation has occurred (Bayet & Nelson, 2020). We can therefore examine potential moderating effects of age on any observed relations between maternal anxiety and infants’ neural responses to emotional faces. Also, we examine not only the NC (as in de Haan et al., 2004, and Taylor-Colls & Pasco Fearon, 2015) but also two additional ERP components related to face- and emotion-processing in infancy: the N290—a negative deflection occurring approximately 290 ms post stimulus onset (e.g., Hoehl & Striano, 2008; Munsters & Kenner, 2017; Jessen & Grossmann, 2015), and the P400—a positive deflection peaking approximately 400 ms post onset of an emotional face (e.g., Leppanen, Mouslon, Vogel-Farely, & Nelson, 2007; Kobiella et al., 2008). The N290 has been associated consistently with face processing, and its amplitude can be modulated by emotional expressions (e.g,. Batty & Taylor, 2006; Faja, Dawson, Aylward, Wijsman, & Webb, 2016; Leppanen et al., 2007; Rigato, Farroni, & Johnson, 2010). The P400 is also associated with face processing as well as allocation of infant attention (Xie & Richards, 2016). Research shows that for both N290 and P400, amplitude is greater during periods of infant attention (versus inattention) (Guy, Zieber, & Richards, 2016).
We hypothesized there would be an association between maternal anxiety and infant neural responses to emotional faces. Given conflicting patterns of results in the limited relevant prior research (e.g., in which higher ratings of positive maternal characteristics were associated with both reduced and enhanced infant neural responses to emotional faces; see de Haan et al., 2004; Taylor-Colls & Pasco Fearon, 2015), we did not posit strong predictions about the directionality of the associations, and thus the present study is necessarily exploratory. However, based on existing research, several specific possible patterns of results may be likely. In line with a “desensitization/habituation” account, infants of highly anxious mothers may show reduced neural responses (i.e., smaller NC, N290, P400 amplitudes) to faces expressing negative emotions (e.g., anger, fear), given research demonstrating that infants of anxious mothers have increased exposure to these types of emotional expressions (Crugnola et al., 2016). These results would be in line with those of de Haan et al. (2004), i.e., that infants of mothers with joyful and positive personalities had smaller NCs to happy versus fearful faces. Alternatively, a “sensitization” account would predict that infants of anxious mothers show enhanced neural responses (i.e., larger NC, N290, P400 amplitudes) to faces depicting negative emotions; such findings would be in line with results from Taylor-Colls and Pasco Fearon (2015), i.e., that infants of mothers’ exhibiting sensitive, non-hostile behaviors had larger NCs to happy relative to neutral faces. Finally, in line with accounts positing that more neural resources are needed to process less frequent or ambiguous stimuli, infants of highly anxious mothers may exhibit enhanced neural responses (i.e., larger NC, N290, P400) to both positive and negative emotional expressions, given research demonstrating that maternal anxiety is characterized not only by a reduction in positive emotional displays but also a general flat affect and reduced emotional tone (Nicol-Harper et al., 2007), making all emotional expressions potentially less familiar and more ambiguous to the infant, and subsequently requiring more neural resources to process. These results would be in line with de Haan et al.’s findings that infants of highly positive mothers showed enhanced NCs to fearful faces. Given age-related differences in development of emotion-processing neural circuitry (Salzwedel et al., 2019) and the fact that infants of different ages may have had more or less exposure to maternal anxiety, we explored infant age as a potential moderator of associations between maternal anxiety and infants’ neural responses to emotional faces. We expect that our findings will guide future research in this understudied area.
Methods
This study is part of a large-scale, longitudinal project examining infant and child neural correlates of emotion-processing. Methods and analyses for the infant ERP data are reported in Xie et al., 2019. Here, we provide brief descriptions of the main methods, and refer readers to Xie et al. for further detail.
Ethical Considerations
The Institutional Review Board approved all methods and procedures used in this study and all parents gave informed consent prior to participation.
Participants
Three hundred and thirty-seven infants and their mothers were recruited from a database of families who had volunteered for research following their infants’ birth. All infants were typically developing, born full-term, and had no pre- or postnatal complications. Infants were assessed at one of three ages: 5 months (N = 113), 7 months (N = 119), or 12 months (N = 105). For the present study, 195 mother-infant dyads were excluded from final analysis: 160 infants did not provide enough useable (artifact-free) EEG data, 15 infants refused to wear the EEG net, 8 infants were later diagnosed with Autism spectrum disorder, 6 mothers did not complete all focal questionnaires for the present study, 3 infants had reported prenatal exposure to opioid or antipsychotic medication, and 3 infants were excluded due to computer/procedural error.
The final sample consisted of 142 infants and their mothers (48 5-month-olds, 23 females; 44 7-month-olds, 22 females; 50 12-month-olds, 25 females). This retention rate of 42% is similar to previous ERP studies of similarly aged infants (Hoehl, 2015, Jessen & Grossmann, 2015; Leppanen et al., 2007). Data retention did not differ by infant age (F(2) = 1.30, p = 0.27). Parent-reported infant race was 83.1% White, 12.7% multi-racial, 2.1% Asian, 0.7% Asian Indian, and 1.4% not reported; 8.4% were Hispanic. Families were predominantly of higher income (73.48% ≥ $100,000/year) and parental education (majority earned a graduate degree). Included and excluded families did not differ on demographic variables (ps > 0.37), maternal anxiety or depression symptom scores (ps > .93), or infant negative emotionality (p = 0.50).
ERP Stimuli and Task Procedure
Stimuli consisted of 150 trials of female faces expressing happy, angry, and fearful emotions (50 trials per emotion category) from the NimStim Face Stimulus Set (Tottenham et al., 2009), presented on a gray background via Eprime 2.0 (Psychology Software Tools). There were five different exemplars (female models) within each emotion category; thus, a single stimulus repeated 10 times throughout the task. Each face stimulus was presented for 1000 ms, preceded by a black center fixation cross that remained on screen until the infant was attending and an experimenter in an adjacent room advanced to the face stimulus (minimum inter-stimulus interval = 700 ms). See Figure 1 for a schematic of the ERP task.
Figure 1.
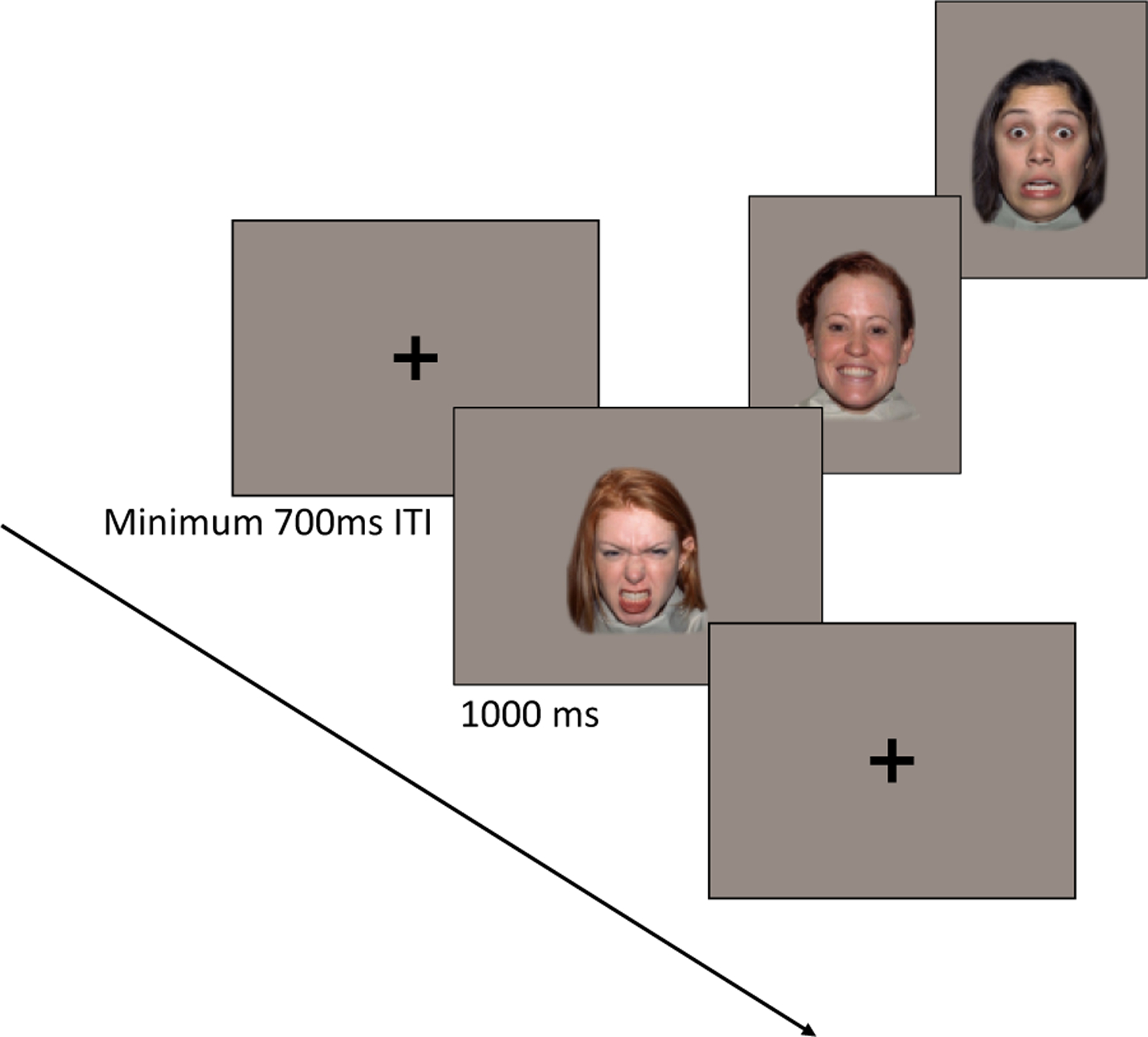
Schematic of ERP task design
Live video feed from the testing room was used to determine when infants were attending to the screen; a second experimenter sat in the testing room next to the infant to direct their attention to the screen as necessary. Infants sat on their caregiver’s lap for the duration of the experiment. Caregivers wore a visor to prevent visual access to the screen. Testing continued for a maximum of 150 trials or until the infant’s attention could no longer be maintained. The number of completed trials did not differ by infant age, F(2,139) = 1.99, p = .141, or by emotion category, F(2, 278) = .80, p = .452. (see Supplemental Information online for average trials completed by age group).
EEG Recording, Processing, and Analysis
Continuous scalp EEG was recorded from a 128-channel HydroCel Geodesic Sensor Net (HCGSN; Electrical Geodesic Inc.), referenced to the vertex electrode (Cz), sampled at 500 Hz. Impedances were kept at or below 100 kilo-ohms. Continuous EEG data were filtered using a 0.3–30 Hz bandpass filter, and segmented to 1200 ms post-stimulus onset with a 100 ms pre-stimulus baseline. Data were baseline-corrected, and automated artifact detection flagged EEG with > 200 microvolt fluctuations within a segment; segments/channels containing eye blinks, eye movements, or drift were flagged by visual inspection. These bad channels were replaced using spherical spline interpolation, although trials were excluded if more than 15% of the channels contained artifact, consistent with previous infant ERP studies (Luyster, Powell, Tager-Flusberg, & Nelson, 2014; Righi, Westerlund, Congdon, Troller-Renfree, & Nelson, 2014). Data were re-referenced to the average reference. Infants who contributed fewer than 9 artifact-free trials per condition were excluded from final analyses (in line with prior publications; Xie et al., 2019). Across ages, infants contributed an average of 21.9 happy, 21.6 angry, and 21.8 fearful usable trials; the number of trials per condition did not differ by age group (F(1,2) = 1.990; p = .141).
ERP Components for Analysis.
We focused on three ERP components based on prior research showing links between each component and face- and emotion-perception (de Haan, et al., 2003; Taylor-Colls & Pasco Fearon, 2015; Leppanen, et al., 2007; Kobiella et al., 2008; Hoehl & Striano, 2008; van den Boomen, Munsters & Kemner, 2017; Jessen & Grossmann, 2015): the NC, N290, and P400. We followed procedures outlined in Xie et al., 2019 to determine time windows and electrode clusters from which to extract all ERP components. In brief, all electrode clusters covered scalp regions commonly associated with NC, N290, and P400 (Leppänen et al., 2007; Peltola, Leppänen, Mäki, & Hietanen, 2009; Rigato et al., 2010; Vanderwert et al., 2015; Xie & Richards, 2016) and were named based on 10–10 electrode positions surrounding the electrodes or the scalp locations covered by the electrodes (Figure 2). Components were extracted in time windows comparable to those used in previous infant emotion-processing research (Kobiella et al., 2008; Leppänen et al., 2007; Vanderwert et al., 2015). The NC was analyzed in four electrode clusters in the frontal and central regions—’FrontalZ,’ ‘CentralZ,’ ‘Central3,’ and ‘Central4.’ The N290 and P400 were analyzed in five electrode clusters in occipital regions—’Occipital-Temporal_L,’ ‘Occipital-Temporal_R,’ ‘Occipital-Inion_L,’ ‘Occipital-Inion_R,’ and ‘Occipital-Inion_Z.’ Mean NC amplitude and mean P400 amplitude were extracted 300–600 ms post stimulus onset. We calculated the mean to better quantify activation in each of these longer components (Xie et al., 2019). Peak N290 amplitude was extracted 190–290 ms post stimulus onset and was corrected for the pre-N290 positive peak by measuring the peak-to-peak amplitude. Specifically, we measured the pre-N290 positive peak amplitude and subtracted it from the N290 peak amplitude for each condition. Using the peak-to-peak amplitude controls for differences at the pre-N290 positive peak and reduces the effect of positive or negative trends in the ERP, both of which may drive N290 amplitude differences between infants (Guy et al., 2016; Kuefner, de Heering, Jacques, Palmero-Soler, & Rossion, 2010; Peykarjou, Pauen, & Hoehl, 2014).
Figure 2.
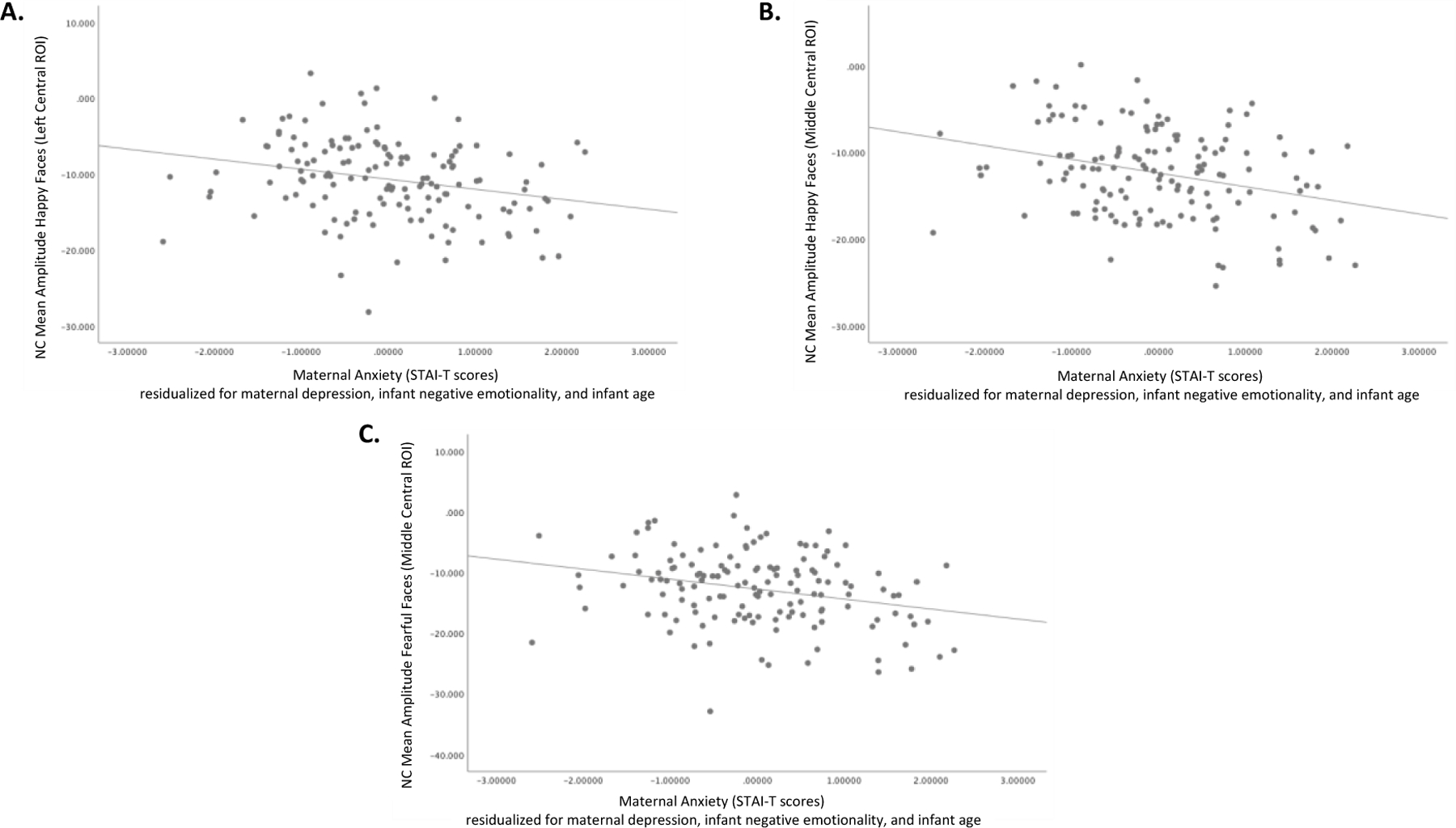
Scatterplots showing the relation between greater maternal anxiety scores (STAI-T) and amplified (more negative) NC to happy faces in the left central ROI (A), happy faces in the middle central ROI (B), and fearful faces in the middle central ROI (C). Analyses controlled for maternal depressive symptoms, infant negative emotionality, and infant age.
Maternal Anxiety
Maternal anxiety was assessed via the Trait Anxiety form of the Spielberger State-Trait Anxiety Inventory (STAI-T; Spielberger, 1983): a 20-item self-report questionnaire designed to measure anxiety proneness by rating the frequency of general mood states (e.g., “I feel nervous and restless”) on a 4-point scale, ranging from “almost never” to “almost always.” Item scores were summed to create a total score (possible range 20–80); higher scores indicate greater anxiety. The STAI is commonly used to assess maternal anxiety in the postpartum period (see Dennis et al., 2017; Leach et al., 2017 for reviews). At the time of publication of the STAI-T, the median alpha coefficient of internal consistency was reported as 0.90, and test-retest coefficients ranged from 0.73 to 0.86 (Barnes, Harp, & Jung, 2002). The majority of mothers completed the questionnaire at home through a secure online link; for mothers in the final sample, the mode for completion was 1 day before their lab visit (range = 30 days prior to lab visit to 14 days post lab visit). A small minority of mothers used laboratory time during the EEG visit to finish questionnaires they had started at home.
Covariates
To investigate the specific association of maternal anxiety with infant neural responses to emotional faces, we assessed maternal depression symptoms, infant negative emotionality, and family SES to consider as covariates in analyses.
Maternal Depression.
Maternal depression symptoms were measured using the Beck Depression Inventory (BDI; Beck, Rush, Shaw, & Emery, 1979), a 21-item self-report questionnaire that assesses the frequency and intensity of depressive symptoms in the past two weeks. Items were scored on a 4-point scale (range 0–3) and summed for a total possible range of 0–63; higher scores indicate greater depression symptoms.
Infant Negative Emotionality.
Infant negative emotionality was assessed via the Negative Emotionality (also referred to as Negative Affectivity) factor score from the Infant Behavior Questionnaire-Revised (IBQ-R; Gartstein & Rothbart, 2003). The IBQ-R is a validated caregiver-report questionnaire of infant temperament and behavior. The Negative Emotionality score is derived by averaging scores of four IBQ-R subscales: Sadness (14-items), Distress to Limitations (16-items), Fear (16-items), and Falling Reactivity (reverse-scored; 13-items). For each subscale, parents reported how often their infant exhibited a given behavior in the past 1–2 weeks, using a 7-point scale ranging from “never” to “always.” Higher scores indicate greater infant negative emotionality.
Family Socioeconomic Status (SES).
A composite variable was created to quantify family SES based on parental self-report of parental educational attainment (ranging from 8th grade education or less to M.D., PhD. J.D. or equivalent) and annual household income. The highest education level reported for either parent was selected to minimize missingness. Education level and household income were standardized and averaged; higher scores indicate higher SES. The utility of using a composite of parental education and income status variables to create a single, family SES variable is described in Caro and Cortes (2012).
Data Analysis Plan
Analyses of Maternal Anxiety and Covariates.
One-way ANOVAs were conducted to test for differences by infant age group in maternal anxiety, maternal depression symptoms, infant negative emotionality, and family SES. Pearson correlations among maternal anxiety, the infant ERP components (NC, N290, and P400), and the covariates were examined to determine the need to control for potential covarying influences of maternal depression, infant negative emotionality, family SES, and infant age in subsequent focal analyses of maternal anxiety associations with infant ERP amplitudes. For preliminary analyses, we adopted the conventional alpha of .05, two-tailed tests.
Focal Analyses between Maternal Anxiety and Infant ERP.
Separate hierarchical regressions were conducted to examine relations between maternal anxiety and infants’ ERPs to emotional faces. Infants’ mean/peak amplitude to each emotion condition (happy, fearful, neutral) for each ERP component (NC, P400, N290) constituted the dependent variables, and maternal anxiety (STAI-T) scores constituted the independent variable. Relevant covariates emerging from correlational analyses were entered together in the first block. To control for multiple comparisons across emotion condition and electrode cluster, we used the following Bonferroni correction formula: α = .05/(NEmotions x Nclusters). There were three emotion conditions. For the N290 and P400 analyses there were 5 clusters, resulting in a corrected alpha of .003. For the NC analyses, there were 4 clusters, resulting in a corrected alpha of .004.
Results
Descriptive Statistics and Analyses
Maternal Anxiety and Covariates.
See Table 1 for descriptive statistics for maternal anxiety and depression symptoms, infant negative emotionality, and family SES by infant age group. These variables did not differ by infant age. Table 2 shows the bivariate correlations among these variables, collapsed across age groups. Given that maternal anxiety was correlated with both maternal depression symptoms and infant negative emotionality, we controlled for each of these variables in focal analyses. Family SES did not correlate with maternal anxiety or any covariates; therefore, we did not consider family SES further.
Table 1.
Descriptive statistics and tests of significant difference across infant age groups for maternal anxiety and depressive symptoms, infant negative emotionality, and family SES (N=142)
| Variable | Infant Age Group | Min | Max | Mean | S.D. | Difference Across Age Groups |
|---|---|---|---|---|---|---|
| Maternal Anxiety Symptoms (STAI-T) | 5 mo | 22 | 45 | 31.87 | 5.81 | F(2, 139) = 2.391, p = .095 |
| 7 mo | 21 | 56 | 35.18 | 8.46 | ||
| 12 mo | 22 | 52 | 33.40 | 7.36 | ||
|
| ||||||
| Maternal Depression Symptoms (BDI) | 5 mo | 0 | 15 | 4.81 | 3.23 | F(2, 139) = 2.827, p = .063 |
| 7 mo | 0 | 27 | 6.53 | 5.44 | ||
| 12 mo | 0 | 14 | 4.73 | 3.37 | ||
|
| ||||||
| Infant Negative Emotionality (IBQ-R) | 5 mo | 1.87 | 4.30 | 2.97 | 0.59 | F(2, 139) = 1.264, p = .286 |
| 7 mo | 1.79 | 4.90 | 3.07 | 0.67 | ||
| 12 mo | 1.85 | 5.08 | 3.19 | 0.74 | ||
|
| ||||||
| Family SES (Parental Income and Education, standardized average) | 5 mo | −1.74 | 0.71 | 0.077 | 0.56 | F(2, 139) = .428, p = .653 |
| 7 mo | −6.30 | 1.03 | −0.01 | 1.21 | ||
| 12 mo | −2.99 | 1.03 | −0.09 | 0.79 | ||
Notes. 5 mo N = 48, 7 mo N = 44, 12 mo N= 50
Table 2.
Pearson correlation coefficients among maternal anxiety symptoms, maternal depression symptoms, infant negative emotionality, and family SES, collapsed across all infant age groups (N=142)
| 1 | 2 | 3 | |
|---|---|---|---|
| 1. Maternal Anxiety Symptoms | __ | ||
| 2. Maternal Depression Symptoms | .583*** | __ | |
| 3. Infant Negative Emotionality | .281** | .086 | __ |
| 4. Family SES | .042 | −.063 | −.110 |
p < .001
p < .01.
Infant Age.
Infant age (in days) was associated with infant neural responses to all emotions for every ERP component examined (see Supplemental Information online for statistics). As infant age increased, amplitude for all ERP components increased for all emotions across a wide range of ROIs, demonstrating developmental changes in infants’ emotion-processing neural system. We thus included infant age as a covariate in focal analyses. We also explored age as a moderator of relations between maternal anxiety and infant ERP amplitudes.
Focal Analyses: Relations between Maternal Anxiety and Infants’ ERPs to Emotional Faces
Focal analyses examined whether maternal anxiety was associated with individual differences in infants’ neural responses to faces. As noted, the N290 and P400 analyses used a Bonferroni corrected alpha of .003 and the NC analyses a Bonferroni corrected alpha of .004.
Maternal anxiety accounted for a significant proportion of variance in infant NC amplitude. Greater maternal anxiety predicted enhanced (more negative) NC amplitude for happy faces in the left central ROI and middle central ROI. The same pattern was found for fearful faces in the middle central ROI. See Tables 3–5. In all three instances, maternal anxiety was the only predictor of infant ERP amplitude to meet the corrected significance criterion. Thus, greater maternal anxiety was associated with enhanced (more negative) NC amplitude to happy and fearful faces, beyond covarying influences of maternal depression symptoms, infant negative emotionality, and infant age (Figures 2 and 3).
Table 3.
Summary of hierarchical regression analysis predicting left central NC mean amplitude for happy faces (N=142)
| Variable | ß | b (SE) | CI95% | t | R | R2 | ΔR2 |
|---|---|---|---|---|---|---|---|
| Block 1 | .233 | .055 | .055 | ||||
| Infant Age (days) | −.211 | −.012 (.005) | [−.022, −.003] | −2.516 | |||
| Infant Neg Emo | −.042 | −.328 (.655) | [−1.624, .968] | −.501 | |||
| Mat Dep Symptoms | .069 | .088 (.106) | [−.122, .297] | .827 | |||
| Block 2 | .341 | .116 | .062* | ||||
| Infant Age (days) | −.196 | −.011 (.005) | [−.021, −.002] | −2.403 | |||
| Infant Neg Emo | .030 | .238 (.662) | [−1.070, 1.546] | .360 | |||
| Mat Dep Symptoms | .250 | .318 (.127) | [.067, .569] | 2.507 | |||
| Maternal Anxiety | −.320 | −.230 (.074) | [−.377, −.083] | −3.100* |
p = .002; Infant Neg Emo = Infant Negative Emotionality; Mat Dep Symptoms = Maternal Depression Symptoms
Table 5.
Summary of hierarchical regression analysis predicting middle central NC mean amplitude for fearful faces (N=142)
| Variable | ß | b (SE) | CI95% | t | R | R2 | ΔR2 |
|---|---|---|---|---|---|---|---|
| Block 1 | .196 | .038 | .038 | ||||
| Infant Age (days) | −.196 | −.013 (.006) | [−.024, −.002] | −2.325 | |||
| Infant Neg Emo | .053 | .476 (.754) | [−1.014, 1.967] | .632 | |||
| Mat Dep Symptoms | −.007 | −.009 (.122) | [−.251, .232] | −.078 | |||
| Block 2 | .335 | .112 | .074* | ||||
| Infant Age (days) | −.180 | −.012 (.005) | [−.023, −.001] | −2.204 | |||
| Infant Neg Emo | .132 | 1.180 (.756) | [−.316, 2.676] | 1.560 | |||
| Mat Dep Symptoms | .191 | .277 (.145) | [−.010, .564] | 1.910 | |||
| Maternal Anxiety | −.349 | −.286 (.085) | [−.453, −.118] | −3.370* |
p = .001; Infant Neg Emo = Infant Negative Emotionality; Mat Dep Symptoms = Maternal Depression Symptoms
Figure 3.
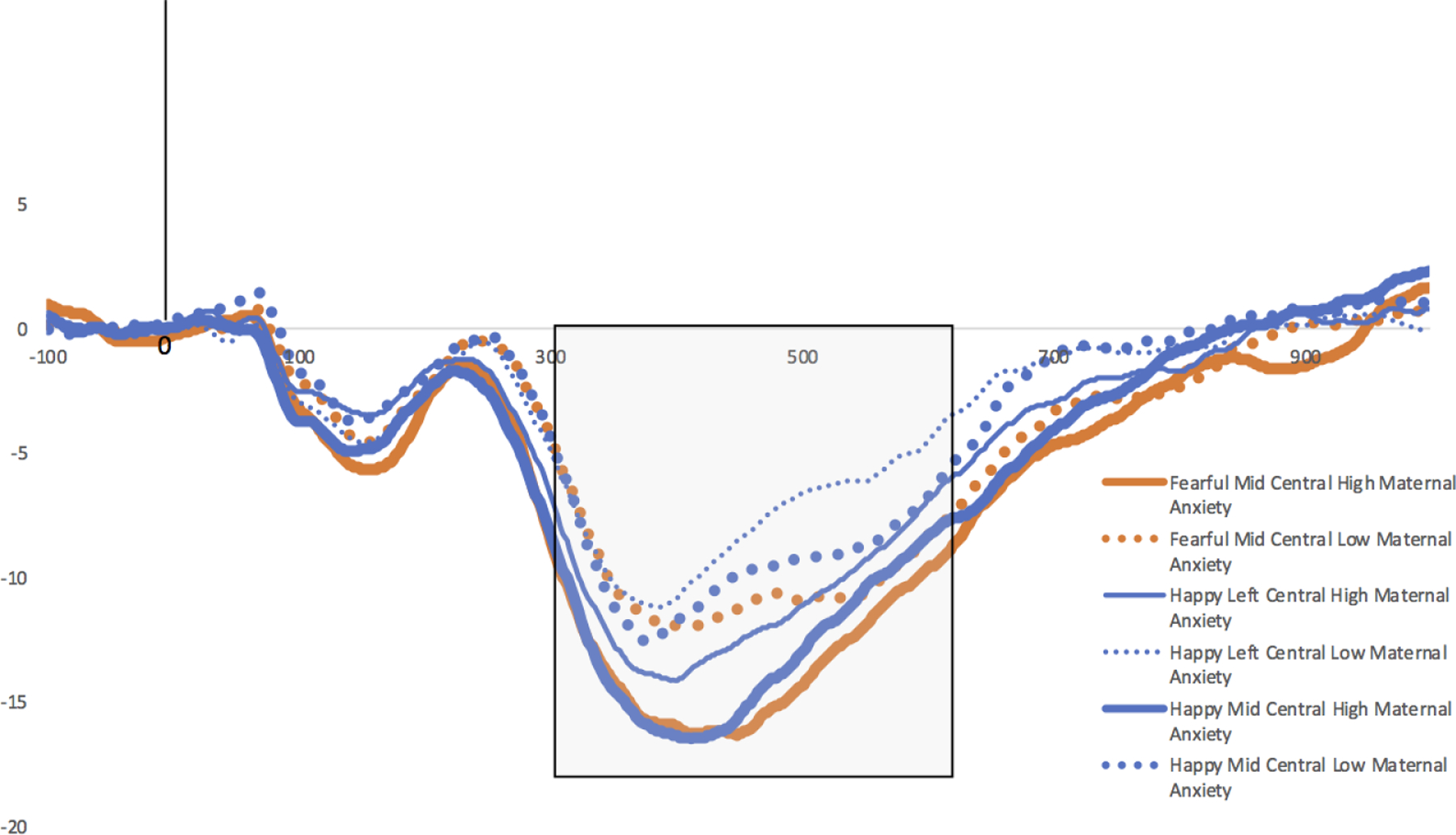
ERP amplitude for the NC in response to fearful faces in the mid-central ROI (thick orange solid and dotted lines), happy faces in the mid-central ROI (thick blue solid and dotted lines), and happy faces in the left-central ROI (thin blue solid and dotted lines). Infants of mothers reporting higher anxiety (solid lines) have more negative NCs compared to infants of mothers reporting lower anxiety (dotted lines) (+/− 2SD STAI scores used for high/low anxiety visualization). The shaded box indicates the period in which mean ERP amplitude was extracted for NC analyses.
Exploring Infant Age and Infant Negative Emotionality as Moderators
Given correlations between infant ERP amplitude and infant age, we explored potential moderating effects of infant age on the relations between maternal anxiety and infant ERPs to emotional faces. All moderation analyses were conducted using the PROCESS custom dialogue add-on in SPSS (Hayes, 2018). Separate analyses were conducted for maternal anxiety predicting ERP amplitude for each component in each condition and ROI, and the same Bonferroni correction formula was applied as described in the methods section. Maternal depression symptoms and infant negative emotionality were entered together as covariates, and age was entered as the moderator. Age was not a significant moderator for any ERP component for any emotion in any ROI as indicated by non-significant changes in R2 for the interaction term ‘maternal anxiety X age’ (ps > .012). Given prior research demonstrating that aspects of infant emotionality moderated relations between maternal personality and infant NC amplitude (de Haan et al., 2004), we also explored infant negative emotionality as a potential moderator of relations between maternal anxiety and infant ERP amplitudes. Moderation analyses were conducted identically to those described above, except age was entered together with maternal depression symptoms as covariates, and infant negative emotionality was entered as the moderator. Here too, no significant results emerged as per non-significant changes in R2 for the interaction term ‘maternal anxiety X infant negative emotionality’ (ps > .012).
Discussion
We examined whether maternal anxiety was associated with typically developing infants’ neural responses to emotional faces (happy, angry, fearful) in the first year of life. We examined this relation with three ERP components, which have each been linked to early face- and emotion-processing: the NC, N290, and P400 (Xie et al., 2019). We found that greater maternal anxiety was associated with an enhanced (more negative) infant NC amplitude to happy and fearful faces, beyond any covarying influences of maternal depression symptoms, infant negative emotionality, and infant age. Maternal anxiety was not associated with NC amplitude to angry faces, or to N290 or P400 amplitude to any emotion. Given the NC’s more specified function of attentional orienting and/or increased attentional arousal to emotional aspects of stimuli (Courchesne, Ganz, & Norcia, 1981; Nelson, 1994; Richards, 2003; de Haan, Johnson & Halit, 2003), results suggest that the relation between maternal anxiety and infant neural responses to emotional faces may be a function of these specific attentional processes, as opposed to broader processes such as general face processing and feature detection, which are associated with the P400 and N290 (de Haan, Pascalis & Johnson, 2002; Halit, de Haan, & Johnson, 2003; Scott & Monesson, 2010; Scott & Nelson, 2006).
Our findings are in line with limited prior research examining associations between maternal psychological/behavioral characteristics and infant neural processing of emotions. Two prior studies found that infants’ neural responses to emotional faces related to maternal positive traits (e.g., positive personality characteristics, sensitive/engaged caregiving: de Haan et al., 2004; Taylor-Colls & Pasco Fearon, 2015), and another study found that infants’ neural responses to emotional vocalizations paired with emotional faces related to prenatal maternal anxiety (Otte et al., 2015). The present study extends this research by demonstrating that infant neural responses are associated with maternal negative emotionality—specifically elevated anxiety—assessed postnatally. Notably, across prior studies and the present study, the NC emerged as the ERP component showing observable differences in infants by maternal characteristics. Our findings also complement the extant behavioral research demonstrating links between maternal anxiety and infants’ and children’s emotion-processing and regulation abilities (Jover et al., 2014; Crugnola et al., 2016, Creswell et al., 2008).
Prior research has shown that children’s neural correlates of emotion-processing are associated with difficulties with emotion regulation and psychopathology, including anxiety (Bechor et al., 2019; Auday et al., 2018). Our findings suggest that maternal anxiety may be one factor that shapes offspring neural processing of emotions, consequently influencing later psychological and emotional well-being. The enhanced NC amplitude observed here in infants of more anxious mothers suggests that increased attentional resources related to the salience, significance, or familiarity of the emotional stimulus (de Haan, Johnson, & Halit, 2003) were recruited to process happy and fearful faces. These attentional proclivities may have downstream consequences for general emotional reactivity and regulation, especially if they become embedded in underlying emotion-processing neural circuitry. Longitudinal examination of these children will determine the long-term impact of these early neural processing patterns on later emotional functioning.
Links between altered attention to emotional stimuli and risk for anxiety have been well-documented in behavioral research (Bar-Haim et al., 2007). Positive relations between postnatal maternal anxiety and heightened attention to emotional faces have also been shown in both infants’ (4 to 24 months; Morales et al., 2017) and in older children (6 to 14 years; Mogg et al., 2016; Montagner et al., 2016). Notably, behavioral findings often demonstrate links between risk factors for anxiety and attentional biases to negative or threatening faces, but not to happy faces. Our findings demonstrate an association between maternal anxiety and greater infant attentional resources to both fearful and happy faces, potentially indicating a difference in sensitivity of the ERP as an index of altered attentional processes associated with anxiety compared to behavioral indices. Future research that combines both behavioral and neural indices of infant attention will be useful in further characterizing the role of infant attention in relation to maternal anxiety.
The mechanisms by which maternal anxiety may influence infant neural processing of emotions are as yet unknown. Several potential pathways are possible. Early life experiences significantly impact development of brain structure and function (Nelson, 2014; Teicher et al., 2016; Dawson, Klinger, Panagiotides, Hill & Spieker, 1992; Jones, Field, & Almeida, 2009; Hane & Fox, 2006; Lupien et al., 2011). Thus, maternal anxiety may influence how infants’ brains respond to relevant social and emotional stimuli in their environment via alterations in maternal caregiving behaviors, which have been shown to be impacted by maternal anxiety (Crugnola et al., 2016; Murray, et al., 2007; Nicol-Harper, et al., 2007; Warren et al., 2003). Infants’ amplified NC responses to happy and fearful faces may be a consequence of differences in the frequency and intensity of emotional expressions that highly anxious mothers express, and may reflect the degree of attentional resources required for infants to process these expressions.
Indeed, our pattern of results is most consistent with the hypothesis that more neural resources are needed to process less frequent or more ambiguous stimuli. Research demonstrates that maternal anxiety is characterized not only by a reduction in positive emotional displays but also generally flat affect and reduced emotional tone (Nicol-Harper et al., 2007). Thus, both positive and negative emotional expressions (i.e., happiness and fear) may be less frequently or intensely displayed and consequently less familiar and more ambiguous to the infant, requiring more neural resources to process, as reflected in the amplified NC to fearful and happy faces observed in our data. These results are in line with de Haan et al.’s (2004) findings that infants of highly positive mothers showed enhanced NCs to fearful faces, which may be less frequently or intensely displayed in highly positive mothers. Notably, we did not find a relation between maternal anxiety and infants’ NC amplitude to angry faces. These expressions may be salient to infants regardless of maternal anxiety status. A limitation of the present study is that we did not directly assess infants’ experiences with maternal emotional expressions. Future research should examine associations between infant neural responding to emotional stimuli and maternal emotional expressions (type, frequency, degree) during mother-infant interactions with mothers who vary in reported anxiety in order to help clarify the role of the emotional environment in shaping individual differences in infants’ neural processing of emotional faces.
Other potential mechanisms include genetic and prenatal processes. Shared genetic factors may account for both maternal anxiety tendencies and infants’ neural processing patterns. Interestingly, the effects of maternal anxiety on infant ERP amplitudes held when controlling for the infant temperament domain of negative emotionality, which has been linked to later internalizing and anxiety problems, including in the current sample (Behrendt, Wade, Bayet, Nelson, & Bosquet Enlow, 2019), and might be, at least in part, genetically driven. Because anxiety commonly emerges or is exacerbated during pregnancy and the postnatal period, and generally shows moderate stability across time (Lovibond, 1998), mothers who demonstrated higher anxiety in the current study also may have experienced heightened anxiety in pregnancy. Maternal anxiety during pregnancy may exert influences in utero on the developing fetus’s brain through various biological disruptions (e.g., elevated glucocorticoid levels; Weinstock, 2005), and existing research has documented relations between prenatal maternal anxiety and changes in infants’ brain function (Otte et al., 2015) and structure (Rifkin-Graboi et al., 2015). Future research should include multiple assessments of maternal anxiety beginning in pregnancy to determine sensitive periods of exposure effects, and assess genetic, epigenetic, and biological factors (e.g., cortisol in pregnancy) to determine potential biological mechanisms of influence. Notably, these hypothesized mechanisms are not mutually exclusive and may have interactive effects on infant brain development.
Although there were clear age effects on ERP amplitude across components, infant age did not moderate the relation between maternal anxiety and infants’ neural responses to emotional faces. A few possibilities could explain this null result. First, maternal anxiety may exert similar effects on infant neural processing regardless of infant age or duration of exposure. Alternatively, if the STAI indexed a stable anxious trait, effects may have occurred early in infancy and remained stable over the first year of life. Longitudinal research is needed to determine when in development exposure to maternal anxiety may influence infant neural processing of emotions, whether such effects are persistent if maternal anxiety remits, and whether extended exposure to maternal anxiety leads to even greater differences in neural processing of emotions.
This study was limited in that the sample was generally high SES and predominantly White, non-Hispanic. Thus, the results may not be widely generalizable, and future research with more diverse samples should be conducted to examine the generality of the relations we observed here. Consideration of other factors that may interact with/amplify maternal anxiety effects (e.g., exposure to stress, availability of social support) will be important. Also, we tested a healthy community sample; findings may differ in a clinical sample with more extreme levels of maternal anxiety. The fact that effects were observed in the current sample suggests that even moderate levels of anxiety may influence infant brain development. Longitudinal studies beginning in pregnancy that assess maternal mental health repeatedly and include measures of a variety of potential mechanisms (e.g., genetic factors, prenatal exposure to cortisol, caregiving quality) will help develop a more comprehensive understanding of how maternal anxiety specifically, and mental health more broadly, influences infant neural processing of emotions and, ultimately, long-term emotional well-being.
Conclusion
We examined whether maternal anxiety was associated with infants’ neural responses to emotional expressions. Results showed that greater maternal anxiety was associated with infants’ enhanced neural responses to happy and fearful faces (indexed by more negative NC amplitude in left- and mid-central scalp regions), beyond covarying influences of maternal depression symptoms, infant negative emotionality, and infant age. Given links between NC amplitude and attentional processes related to salience, significance, and/or familiarity of the emotional stimulus (de Haan, et al., 2003), results suggest that infants of more highly anxious mothers may need additional attentional resources to process happy and fearful faces (expressions less likely experienced in mother-infant interactions). Future research should investigate mechanisms underlying this association, given possibilities include experiential, genetic, and prenatal factors.
Supplementary Material
Table 4.
Summary of hierarchical regression analysis predicting middle central NC mean amplitude for happy faces (N=142)
| Variable | ß | b (SE) | CI95% | t | R | R2 | ΔR2 |
|---|---|---|---|---|---|---|---|
| Block 1 | .105 | .011 | .011 | ||||
| Infant Age (days) | −.101 | −.006 (.005) | [−.015, .004] | −1.182 | |||
| Infant Neg Emo | −.023 | .178 (.655) | [−1.117, 1.473] | .271 | |||
| Mat Dep Symptoms | .024 | .030 (.106) | [−.180, .239] | .279 | |||
| Block 2 | .322 | .103 | .092* | ||||
| Infant Age (days) | −.083 | −.005 (.005) | [−.014, .005] | −2.403 | |||
| Infant Neg Emo | .112 | .853 (.651) | [−.435, 2.141] | 1.309 | |||
| Mat Dep Symptoms | .245 | .304 (.125) | [.057, .552] | 2.437 | |||
| Maternal Anxiety | −.391 | −.274 (.073) | [−.419, −.130] | −3.755* |
p < .001; Infant Neg Emo = Infant Negative Emotionality; Mat Dep Symptoms = Maternal Depression Symptoms
Key Points.
Exposure to maternal anxiety is associated with alterations in how infants attend to and display emotions, which are linked to later development of emotion-processing problems
Neural circuitry for face- and emotion-processing develops during infants’ first year, and thus postnatal maternal anxiety may affect infants’ neural processing of emotional expressions, but this relation has not yet been tested
Results show that infants of mothers reporting heightened trait anxiety have enhanced neural responses to fearful and happy faces
This link between maternal anxiety and infants’ neural processing of emotional expressions could indicate altered attentional processes that may have downstream consequences for development of internalizing problems
Acknowledgements
This research was supported by grants from National Institute of Health (MH078829) and the Tommy Fuss Center for Neuropsychiatric Disease Research and the Program for Behavioral Science, Department of Psychiatry, Boston Children’s Hospital. Study data were collected and managed using Research Electronic Data Capture (REDCap) tools hosted at Boston Children’s Hospital. We extend our gratitude to Cailin Daley, who was involved in early stages of the project. We are extremely grateful for the parents and infants who participated in this study, without whom this research would not be possible.
Abbreviations:
- EEG
electroencephalogram
- ERP
event-related potentia
- NC
negative central
- STAI-T
Spielberger State-Trait Anxiety Inventory
- BDI
Beck Depression Inventory
- SES
socio-economic status
- IBQ-R
Infant Behavior Questionnaire
Footnotes
Conflicts of Interest
There are no conflicts of interest to report.
References
- Auday ES, Taber-Thomas BC, & Pérez-Edgar KE (2018). Neural correlates of attention bias to masked facial threat cues: Examining children at-risk for social anxiety disorder. NeuroImage: Clinical, 19, 202–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, & Van Ijzendoorn MH (2007). Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychological bulletin, 133(1), 1. [DOI] [PubMed] [Google Scholar]
- Barker ED, Jaffee SR, Uher R, & Maughan B (2011). The contribution of prenatal and postnatal maternal anxiety and depression to child maladjustment. Depression and anxiety, 28(8), 696–702. [DOI] [PubMed] [Google Scholar]
- Batty M, & Taylor MJ (2006). The development of emotional face processing during childhood. Developmental science, 9(2), 207–220. [DOI] [PubMed] [Google Scholar]
- Bayet L, & Nelson CA (2020). The neural architecture and developmental course of face processing. In Rakic P & Rubenstein J (Eds.), Comprehensive Developmental Neuroscience, 2nd Edition. San Diego, CA: Elsevier Press. [Google Scholar]
- Bayet L, & Nelson CA (2019). The perception of facial emotion in typical and atypical development. In Handbook of emotional development (pp. 105–138). Springer, Cham. [Google Scholar]
- Bechor M, Ramos ML, Crowley MJ, Silverman WK, Pettit JW, & Reeb-Sutherland BC (2019). Neural correlates of attentional processing of threat in youth with and without anxiety disorders. Journal of abnormal child psychology, 47(1), 119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Rush AJ, Shaw BF, & Emery G, (1979). Cognitive therapy of depression New York: Guilford Press. [Google Scholar]
- Beebe B, Steele M, Jaffe J, Buck KA, Chen H, Cohen P, … & Feldstein S (2011). Maternal anxiety symptoms and mother–infant self‐and interactive contingency. Infant Mental Health Journal, 32(2), 174–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrendt HF, Wade M, Bayet L, Nelson CA, & Bosquet Enlow M (2019). Pathways to social-emotional functioning in the preschool period: The role of child temperament and maternal anxiety in boys and girls. Development and psychopathology, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro DH, & Cortés D (2012). Measuring family socioeconomic status: An illustration using data from PIRLS 2006. IERI Monograph Series Issues and Methodologies in Large-Scale Assessments, 5, 9–33. [Google Scholar]
- Conte S, Richards JE, Guy MW, Xie W, & Roberts JE (2020). Face-sensitive brain responses in the first year of life. NeuroImage, 211, 116602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Ganz L, & Norcia AM (1981). Event-related brain potentials to human faces in infants. Child development, 804–811. [PubMed] [Google Scholar]
- Creswell C, Woolgar M, Cooper P, Giannakakis A, Schofield E, Young AW, & Murray L (2008). Processing of faces and emotional expressions in infants at risk of social phobia. Cognition and Emotion, 22(3), 437–458. [Google Scholar]
- Crugnola CR, Ierardi E, Ferro V, Gallucci M, Parodi C, & Astengo M (2016). Mother-infant emotion regulation at three months: the role of maternal anxiety, depression and parenting stress. Psychopathology, 49(4), 285–294. [DOI] [PubMed] [Google Scholar]
- Dawson G, Klinger LG, Panagiotides H, Hill D, & Spieker S (1992). Frontal lobe activity and affective behavior of infants of mothers with depressive symptoms. Child development, 63(3), 725–737. [PubMed] [Google Scholar]
- De Haan M, Belsky J, Reid V, Volein A, & Johnson MH (2004). Maternal personality and infants’ neural and visual responsivity to facial expressions of emotion. Journal of Child Psychology and Psychiatry, 45(7), 1209–1218. [DOI] [PubMed] [Google Scholar]
- De Haan M, Johnson MH, & Halit H (2003). Development of face-sensitive event-related potentials during infancy: a review. International Journal of Psychophysiology, 51(1), 45–58. [DOI] [PubMed] [Google Scholar]
- Dean DC, Planalp EM, Wooten W, Kecskemeti SR, Adluru N, Schmidt CK, … & Styner MA (2018). Association of prenatal maternal depression and anxiety symptoms with infant white matter microstructure. JAMA pediatrics, 172(10), 973–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis CL, Falah-Hassani K, & Shiri R (2017). Prevalence of antenatal and postnatal anxiety: systematic review and meta-analysis. The British Journal of Psychiatry, 210(5), 315–323. [DOI] [PubMed] [Google Scholar]
- Dennis TA, Malone MM, & Chen CC (2009). Emotional face processing and emotion regulation in children: An ERP study. Developmental neuropsychology, 34(1), 85–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faja S, Dawson G, Aylward E, Wijsman EM, & Webb SJ (2016). Early event-related potentials to emotional faces differ for adults with autism spectrum disorder and by serotonin transporter genotype. Clinical Neurophysiology, 127(6), 2436–2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R, Granat A, Pariente C, Kanety H, Kuint J, & Gilboa-Schechtman E (2009). Maternal depression and anxiety across the postpartum year and infant social engagement, fear regulation, and stress reactivity. Journal of the American Academy of Child & Adolescent Psychiatry, 48(9), 919–927. [DOI] [PubMed] [Google Scholar]
- Field T (2017). Postpartum anxiety prevalence, predictors and effects on child development: A review. Journal of Psychiatry and Psychiatric Disorders, 1(2), 86–102. [Google Scholar]
- Gartstein MA, & Rothbart MK (2003). Studying infant temperament via the revised infant behavior questionnaire. Infant Behavior and Development, 26(1), 64–86. [Google Scholar]
- Gustafsson HC, Grieve PG, Werner EA, Desai P, & Monk C (2018). Newborn electroencephalographic correlates of maternal prenatal depressive symptoms. Journal of developmental origins of health and disease, 9(4), 381–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy MW, Zieber N, & Richards JE (2016). The cortical development of specialized face processing in infancy. Child Development, 87(5), 1581–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haan MD, Pascalis O, & Johnson MH (2002). Specialization of neural mechanisms underlying face recognition in human infants. Journal of cognitive neuroscience, 14(2), 199–209. [DOI] [PubMed] [Google Scholar]
- Halit H, De Haan M, & Johnson MH (2003). Cortical specialisation for face processing: face-sensitive event-related potential components in 3-and 12-month-old infants. Neuroimage, 19(3), 1180–1193. [DOI] [PubMed] [Google Scholar]
- Hane AA, & Fox NA (2006). Ordinary variations in maternal caregiving influence human infants’ stress reactivity. Psychological science, 17(6), 550–556. [DOI] [PubMed] [Google Scholar]
- Hayes AF (2018). Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach (2nd Ed). New York: The Guilford Press. [Google Scholar]
- Henrichs J, Schenk JJ, Schmidt HG, Velders FP, Hofman A, Jaddoe VW, … & Tiemeier H (2009). Maternal pre‐and postnatal anxiety and infant temperament. The generation R study. Infant and Child Development: An International Journal of Research and Practice, 18(6), 556–572. [Google Scholar]
- Hoehl S (2015). How do neural responses to eyes contribute to face-sensitive ERP components in young infants? A rapid repetition study. Brain and cognition, 95, 1–6. [DOI] [PubMed] [Google Scholar]
- Hoehl S, & Striano T (2008). Neural processing of eye gaze and threat‐related emotional facial expressions in infancy. Child development, 79(6), 1752–1760. [DOI] [PubMed] [Google Scholar]
- Jessen S, & Grossmann T (2015). Neural signatures of conscious and unconscious emotional face processing in human infants. Cortex, 64, 260–270. [DOI] [PubMed] [Google Scholar]
- Jones NA, Field T, & Almeida A (2009). Right frontal EEG asymmetry and behavioral inhibition in infants of depressed mothers. Infant Behavior and Development, 32(3), 298–304. [DOI] [PubMed] [Google Scholar]
- Jover M, Colomer J, Carot JM, Larsson C, Bobes MT, Ivorra JL, … & Sanjuan J (2014). Maternal Anxiety following Delivery, Early Infant Temperament and Motheŕ s Confidence in Caregiving. The Spanish journal of psychology, 17. [DOI] [PubMed] [Google Scholar]
- Kobiella A, Grossmann T, Reid VM, & Striano T (2008). The discrimination of angry and fearful facial expressions in 7-month-old infants: An event-related potential study. Cognition and Emotion, 22(1), 134–146. [Google Scholar]
- Kuefner D, De Heering A, Jacques C, Palmero-Soler E, & Rossion B (2010). Early visually evoked electrophysiological responses over the human brain (P1, N170) show stable patterns of face-sensitivity from 4 years to adulthood. Frontiers in human neuroscience, 3, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach LS, Poyser C, & Fairweather‐Schmidt K (2017). Maternal perinatal anxiety: A review of prevalence and correlates. Clinical Psychologist, 21(1), 4–19. [Google Scholar]
- Leppänen JM, & Nelson CA (2009). Tuning the developing brain to social signals of emotions. Nature Reviews Neuroscience, 10(1), 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppänen JM, Moulson MC, Vogel‐Farley VK, & Nelson CA (2007). An ERP study of emotional face processing in the adult and infant brain. Child development, 78(1), 232–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovibond PF (1998). Long-term stability of depression, anxiety, and stress syndromes. Journal of abnormal psychology, 107(3), 520. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Parent S, Evans AC, Tremblay RE, Zelazo PD, Corbo V, … & Séguin JR (2011). Larger amygdala but no change in hippocampal volume in 10-year-old children exposed to maternal depressive symptomatology since birth. Proceedings of the National Academy of Sciences, 108(34), 14324–14329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyster RJ, Powell C, Tager-Flusberg H, & Nelson CA (2014). Neural measures of social attention across the first years of life: Characterizing typical development and markers of autism risk. Developmental Cognitive Neuroscience, 8, 131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinos M, Matheson A, & de Haan M (2012). Links between infant temperament and neurophysiological measures of attention to happy and fearful faces. Journal of Child Psychology and Psychiatry, 53(11), 1118–1127. [DOI] [PubMed] [Google Scholar]
- Mogg K, Wilson KA, Hayward C, Cunning D, Bradley BP (2012). Attentional biases for threat in at-risk daughters and mothers with lifetime panic disorder. Journal of Abnormal Psychology, 121, 852–862. [DOI] [PubMed] [Google Scholar]
- Montagner R, Mogg K, Bradley BP, Pine DS, Czykiel MS, Miguel EC, … Salum GA (2016). Attentional bias to threat in children at-risk for emotional disorders: Role of gender and type of maternal emotional disorder. European Child & Adolescent Psychiatry, 25, 735–742. [DOI] [PubMed] [Google Scholar]
- Morales S, Brown KM, Taber-Thomas BC, LoBue V, Buss KA, Pérez-Edgar KE (2017). Maternal anxiety predicts attentional bias towards threat in infancy. Emotion, 17, 874–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray L, Cooper P, Creswell C, Schofield E, & Sack C (2007). The effects of maternal social phobia on mother–infant interactions and infant social responsiveness. Journal of Child Psychology and Psychiatry, 48(1), 45–52. [DOI] [PubMed] [Google Scholar]
- Nelson CA (1994). Neural correlates of recognition memory in the first postnatal year
- Nelson CA (2014). Romania’s abandoned children Harvard University Press. [Google Scholar]
- Nicol-Harper R, Harvey AG, & Stein A (2007). Interactions between mothers and infants: Impact of maternal anxiety. Infant Behavior and Development, 30(1), 161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor TG, Heron J, Golding J, Beveridge M, & Glover V (2002). Maternal antenatal anxiety and children’s behavioural/emotional problems at 4 years: Report from the Avon Longitudinal Study of Parents and Children. The British Journal of Psychiatry, 180(6), 502–508. [DOI] [PubMed] [Google Scholar]
- O’Toole LJ, DeCicco JM, Berthod S, & Dennis TA (2013). The N170 to angry faces predicts anxiety in typically developing children over a two-year period. Developmental neuropsychology, 38(5), 352–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otte RA, Donkers FCL, Braeken MAKA, & Van den Bergh BRH (2015). Multimodal processing of emotional information in 9-month-old infants II: prenatal exposure to maternal anxiety. Brain and Cognition, 95, 107–117. [DOI] [PubMed] [Google Scholar]
- Peltola MJ, Leppänen JM, Mäki S, & Hietanen JK (2009). Emergence of enhanced attention to fearful faces between 5 and 7 months of age. Social cognitive and affective neuroscience, 4(2), 134–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peykarjou S, Pauen S, & Hoehl S (2014). How do 9‐month‐old infants categorize human and ape faces? A rapid repetition ERP study. Psychophysiology, 51(9), 866–878. [DOI] [PubMed] [Google Scholar]
- Richards JE (2003). Attention affects the recognition of briefly presented visual stimuli in infants: An ERP study. Developmental Science, 6(3), 312–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigato S, Farroni T, & Johnson MH (2010). The shared signal hypothesis and neural responses to expressions and gaze in infants and adults. Social Cognitive and Affective Neuroscience, 5(1), 88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkin-Graboi A, Meaney MJ, Chen H, Bai J, Hameed WBR, Tint MT, … & Qiu A (2015). Antenatal maternal anxiety predicts variations in neural structures implicated in anxiety disorders in newborns. Journal of the American Academy of Child & Adolescent Psychiatry, 54(4), 313–321. [DOI] [PubMed] [Google Scholar]
- Righi G, Westerlund A, Congdon EL, Troller-Renfree S, & Nelson CA (2014). Infants’ experience-dependent processing of male and female faces: Insights from eye tracking and event-related potentials. Developmental cognitive neuroscience, 8, 144–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzwedel AP, Stephens RL, Goldman BD, Lin W, Gilmore JH, & Gao W (2019). Development of amygdala functional connectivity during infancy and its relationship with 4-year behavioral outcomes. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 4(1), 62–71. 10.1016/j.bpsc.2018.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott LS, & Monesson A (2010). Experience-dependent neural specialization during infancy. Neuropsychologia, 48(6), 1857–1861. [DOI] [PubMed] [Google Scholar]
- Scott LS, & Nelson CA (2006). Featural and configural face processing in adults and infants: A behavioral and electrophysiological investigation. Perception, 35(8), 1107–1128. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, & Jacobs GA (1983). Manual for the State-Trait Anxiety Inventory Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- Taylor-Colls S, & Pasco Fearon RM (2015). The effects of parental behavior on infants’ neural processing of emotion expressions. Child Development, 86(3), 877–888. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Samson JA, Anderson CM, & Ohashi K (2016). The effects of childhood maltreatment on brain structure, function and connectivity. Nature Reviews Neuroscience, 17(10), 652. [DOI] [PubMed] [Google Scholar]
- Tomalski P, Moore DG, Ribeiro H, Axelsson EL, Murphy E, Karmiloff‐Smith A, … & Kushnerenko E (2013). Socioeconomic status and functional brain development–associations in early infancy. Developmental Science, 16(5), 676–687. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, … & Nelson C (2009). The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry research, 168(3), 242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Boomen C, Munsters NM, & Kemner C (2017). Emotion-processing in the infant brain: The importance of local information. Neuropsychologia, 10.1016/j.neuropsychologia.2017.09.006 [DOI] [PubMed] [Google Scholar]
- van Heyningen T, Honikman S, Myer L, Onah MN, Field S, & Tomlinson M (2017). Prevalence and predictors of anxiety disorders amongst low-income pregnant women in urban South Africa: a cross-sectional study. Archives of women’s mental health, 20(6), 765–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwert RE, Marshall PJ, Nelson CA III, Zeanah CH, & Fox NA (2010). Timing of intervention affects brain electrical activity in children exposed to severe psychosocial neglect. PLoS One, 5(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren SL, Gunnar MR, Kagan J, Anders TF, Simmens SJ, Rones M, … & Sroufe AL (2003). Maternal panic disorder: infant temperament, neurophysiology, and parenting behaviors. Journal of the American Academy of Child & Adolescent Psychiatry, 42(7), 814–825. [DOI] [PubMed] [Google Scholar]
- Weinstock M (2005). The potential influence of maternal stress hormones on development and mental health of the offspring. Brain, behavior, and immunity, 19(4), 296–308. [DOI] [PubMed] [Google Scholar]
- Xie W, & Richards JE (2016). Effects of interstimulus intervals on behavioral, heart rate, and event‐related potential indices of infant engagement and sustained attention. Psychophysiology, 53(8), 1128–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W, McCormick SA, Westerlund A, Bowman LC, & Nelson CA (2019). Neural correlates of facial emotion-processing in infancy. Developmental science, 22(3), e12758. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


