Abstract
There are > 18 distinct disease-modifying therapy (DMT) options covering 10 mechanisms of action currently approved by the US Food and Drug Administration for the treatment of relapsing–remitting multiple sclerosis (RRMS). Given the multitude of available treatment options, and recent international consensus guidelines offering differing recommendations, there is broad heterogeneity in how the DMTs are used in clinical practice. Choosing a DMT for newly diagnosed patients with MS is currently a topic of significant debate in MS care. Historically, an escalation approach to DMT was used for newly diagnosed patients with RRMS. However, the evidence for clinical benefits of early treatment with high-efficacy therapies (HETs) in this population is emerging. In this review, we provide an overview of the DMT options and MS treatment strategies, and discuss the clinical benefits of HETs (including ofatumumab, ocrelizumab, natalizumab, alemtuzumab, and cladribine) in the early stages of MS, along with safety concerns associated with these DMTs. By minimizing the accumulation of neurological damage early in the disease course, early treatment with HETs may enhance long-term clinical outcomes over the lifetime of the patient.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40263-022-00965-7.
Plain Language Summary
Disease-modifying therapies (DMTs) can help people with multiple sclerosis (MS) by changing the way that their MS develops over time. Some people with MS have relapses when their symptoms get worse, followed by recovery when their MS is remitting. This is called relapsing–remitting MS (RRMS). DMTs can reduce both the number and the severity of relapses. They can also delay the nerve damage that relapses cause. A range of DMTs are approved for treating people with RRMS. These treatments work in different ways, and international treatment guidelines vary on their recommendations for using DMTs in the clinic. Selecting DMTs for people with newly diagnosed RRMS is still a topic of discussion. Previously, people with RRMS only received the more effective high-efficacy therapies (HETs) if their first treatment was not effective. HETs include ofatumumab, ocrelizumab, natalizumab, alemtuzumab, and cladribine. Recently, using HETs at an earlier stage has shown promising results. In this review article, we provide an overview of the clinical strategies and the DMT options that are available for people with MS. Additionally, we discuss the benefits of using HETs for people with newly diagnosed MS and consider the safety issues related to DMTs. We summarize that using HETs to reduce the buildup of nerve damage during the early stages of MS may lead to improved long-term clinical outcomes over a person’s lifetime.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40263-022-00965-7.
Key Points
| Neurological damage begins in the early stages of multiple sclerosis, and may even precede clinically evident symptoms. |
| Early treatment with high-efficacy therapies may enhance long-term clinical outcomes by minimizing the accumulation of neurological damage that occurs in the early stages of disease. |
| Classification of multiple sclerosis disease-modifying therapies as ‘high-efficacy’ versus ‘moderate’ or ‘low-efficacy’ varies between studies; in this review, we classify the following disease-modifying therapies as high-efficacy therapies: ofatumumab, ocrelizumab, natalizumab, alemtuzumab, and cladribine. |
Introduction
Multiple sclerosis (MS) is a chronic, immune-mediated, neurodegenerative disease that causes accumulating damage to the central nervous system (CNS). It can lead to significant neurological disability, particularly in untreated individuals [1, 2]. Currently, there are over 18 distinct disease-modifying therapy (DMT) options covering 10 mechanisms of action currently approved by the US Food and Drug Administration (FDA) and the European Medicines Agency for the treatment of relapsing forms of MS (RMS) [3–6]. Given the multitude of available treatment options and recent international consensus guidelines offering differing recommendations [7, 8], there is broad heterogeneity in how DMTs are used in clinical practice [9].
Choosing a DMT for newly diagnosed patients with MS is currently a topic of significant debate in MS care. Most patients (85–90%) present with relapsing–remitting MS (RRMS) at disease onset [10–12]. Historically, an escalation approach to DMT was used for this population, but the evidence for early high-efficacy therapy (HET) is emerging. Therefore, in this review, we discuss the rationale and evidence for use of the early HET approach in newly diagnosed patients with RRMS.
Early Window of Opportunity in MS
Neurological damage begins in the early stages of MS, prior to manifestation of clinical MS symptoms, as evidenced by elevated levels of serum neurofilament light proteins detected several years prior to clinical presentation [13]. Moreover, accelerated brain atrophy has been observed in asymptomatic individuals with radiologically isolated syndrome [14–16], as well as in patients with clinically isolated syndrome (CIS) [17, 18].
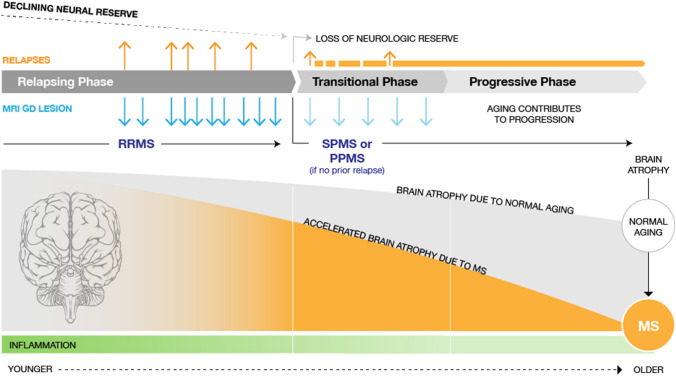
Once MS is clinically evident, focal inflammation dominates the early phase of disease. This MS-related inflammation is most evident in younger patients and decreases with age (Fig. 1). In RRMS, some inflammatory attacks in the CNS cause clearly defined neurological symptoms (relapses), followed by periods of full or partial recovery from new or existing symptoms (remissions) [12]. In addition to these clinically apparent events, clinically silent inflammation contributes to neuronal loss, axon destruction, and demyelination, ultimately leading to irreversible neurologic disability [19]. Accelerated brain atrophy during early inflammatory disease may not initially be associated with noticeable neurologic worsening. This largely subclinical manifestation of disease activity may be due to the brain’s capacity to compensate for a certain degree of neuronal injury and recover from relapses, as attributed to the concept of ‘neurological reserve.’ Upon loss of reserve due to MS and normal aging, the ensuing effects of brain/neuronal loss are unmasked, presenting as the onset of a progressive phase of MS (Fig. 1) [20]. Early termination of the immune attack on the CNS with DMTs may therefore enhance long-term clinical outcomes by minimizing the accumulation of neurological damage that may occur early in the disease course. MS symptoms, accrual of tissue damage, and prognosis vary substantially between patients [21, 22], and there are clearly responders and non-responders to therapy currently on the market [23–25]. Although a personalized approach to management is appropriate, at present it is not possible to predict the responder/non-responder status of individuals without having them try (and potentially fail) multiple MS drugs [26, 27]. Each drug failure can add cumulative neurologic disability, fueling the discussion about the most appropriate strategy to select a treatment for newly diagnosed patients [26].
Fig. 1.
Natural history of MS. Gd gadolinium, MRI magnetic resonance imaging, MS multiple sclerosis, PPMS primary progressive multiple sclerosis, RRMS relapsing–remitting multiple sclerosis, SPMS secondary progressive multiple sclerosis
Treatment Goals and Shared Decision-Making for Patient-Centric MS Care
Given the ongoing neurological damage from the early stages of MS, initial treatment with effective DMTs and close monitoring are crucial to slow/stop disease progression over the lifetime of the patient. Treatment goals have evolved to include treat-to-target strategies that monitor clinical and neuroimaging disease activity beyond clinical relapses [28]. With the emergence of highly efficacious DMTs, no evidence of disease activity (NEDA) is emerging as a popular clinical outcome measurement [28]. NEDA-3 is a composite of three measures of disease activity: no clinical relapses; no confirmed disability progression as measured by the Expanded Disability Status Scale (EDSS); and no new magnetic resonance imaging (MRI) lesion activity [28, 29]. While NEDA captures confirmed disability worsening as measured by the EDSS, it remains strongly focused on the inflammatory component of the disease and is thought to be insensitive to neurodegenerative processes that may be more relevant to the long-term outcomes of people with MS. It has been proposed to expand the definition of NEDA to address microscopic injury and incorporate additional parameters, such as brain atrophy (NEDA-4) and/or neurofilament light protein levels in cerebrospinal fluid or blood (NEDA-5); however, these parameters are not yet established for routine clinical use [28, 29].
Understanding and incorporating individual patient goals and values into treatment decisions is another important component of patient-centered MS care [30]. A patient survey study found that patients prioritized preserving brain health (memory, thinking, brain) over physical disability concerns (walking, strength, vision) [31]. Treatment efficacy and the likelihood of serious side effects were the most important considerations for these patients when choosing a DMT. These findings highlight the need for routine assessment of non-physical MS symptoms, including cognitive and mental health issues and the importance of using shared decision-making to personalize MS care [32, 33].
MS DMT Options and Treatment Strategies
DMT Options (High-Efficacy DMTs or Low/Moderate-Efficacy DMTs)
The > 18 immunomodulatory DMTs currently available for MS treatment in the US and Europe are shown in Table 1 [4–6, 34–36]. The following DMTs are approved in the US and Europe for the treatment of all RMS, including CIS, RRMS, and, for some DMTs, active secondary progressive MS (SPMS): anti-CD20 monoclonal antibodies (subcutaneous ofatumumab and intravenous ocrelizumab), natalizumab (an integrin receptor antagonist administered intravenously), sphingosine 1-phosphate (S1P) receptor modulators (fingolimod, siponimod, ozanimod, ponesimod, all taken orally), fumarates (dimethyl, monomethyl, diroximel, all taken orally), interferons (IFNβ-1b administered subcutaneously, and IFNβ-1a and pegylated IFNβ-1a administered subcutaneously or via intramuscular injection), teriflunomide (oral), and glatiramer acetate (subcutaneous injection). Ocrelizumab is also approved for the treatment of primary progressive MS (PPMS) [37]. An intravenous formulation of ofatumumab is approved for the treatment of chronic lymphocytic leukemia [38]; however, only the subcutaneous formulation will be reviewed in this article. Rituximab, another anti-CD20 monoclonal antibody, is approved for multiple indications in oncology and rheumatology [39] and has long been used as an off-label MS therapy [40]. Alemtuzumab (an intravenously administered CD52-directed cytolytic monoclonal antibody) and cladribine (a purine antimetabolite taken orally) are indicated for the treatment of RRMS and active SPMS; due to their safety profiles, alemtuzumab and cladribine are not recommended for use in patients with CIS [41, 42].
Table 1.
Disease-modifying therapies approved by the US Food and Drug Administration and the European Medicines Agency for multiple sclerosis treatment [4–6, 34–36]
| Drug name | Brand name | Mechanism of action | Route of administration | Approved US indication | US approval year | EU approval year |
|---|---|---|---|---|---|---|
| Infusion/monoclonal therapies | ||||||
| Ofatumumab | Kesimpta® | Anti-CD20 mAb | SC | RMSb | 2020 | 2021 |
| Ocrelizumab | Ocrevus® | Anti-CD20 mAb | IV | RMS or PPMS | 2017 | 2018 |
| Alemtuzumab | Lemtrada® | CD52-directed cytolytic mAb | IV | RMSb | 2014 | 2013 |
| Natalizumab | Tysabri® | Integrin receptor antagonist | IV | RMS; CD | 2004 | 2006 |
| Mitoxantronea | Novantrone® | Synthetic antineoplastic anthracenedione | IV | RMSc | 2000 | 1998 |
| Oral medications | ||||||
| Ponesimod | Ponvory® | S1P receptor modulator | Oral | RMSb | 2021 | 2021 |
| Ozanimod | Zeposia® | S1P receptor modulator | Oral | RMSb | 2020 | 2020 |
| Siponimod | Mayzent® | S1P receptor modulator | Oral | RMSb | 2019 | 2020 |
| Cladribine | Mavenclad® | Purine antimetabolite | Oral | RMSd | 2019 | 2017 |
| Dimethyl fumarate | Tecfidera® | Unknown | Oral | RMS | 2013 | 2014 |
| Generics | 2020 | 2022 | ||||
| Monomethyl fumarate | Bafiertam® (US) | Unknown | Oral | RMSb | 2013 | – |
| Diroximel fumarate | Vumerity® | Unknown | Oral | RMSb | 2013 | 2021 |
| Teriflunomide | Aubagio® | Pyrimidine synthesis inhibitor | Oral | RMS | 2012 | 2013 |
| Fingolimod | Gilenya® | S1P receptor modulator | Oral | RMS | 2010 | 2011 |
| Injectable therapies | ||||||
| Glatiramer acetate | Generic | Unknown | SC | RMSb | 2017 | 2016 |
| Glatopa® (US) | RMS | 2015 | – | |||
| Copaxone® | RMSb | 1996 | 2004 | |||
| Pegylated IFNβ-1a | Plegridy® | Unknown | IM | RMSb | 2021 | 2020 |
| SC | 2014 | 2014 | ||||
| IFNβ-1b |
Betaseron® (US) Betaferon® (EU) |
Unknown | SC | RMS | 1993 | 1995 |
| Extavia® | Unknown | RMSb | 2009 | 2008 | ||
| IFNβ-1a | Rebif® | Unknown | SC | RMS | 1998 | 1998 |
| Avonex® | Unknown | IM | 1996 | 1997 | ||
CD Crohn's disease, EU European Union, IFN interferon, IM intramuscular injection, IV intravenous, mAb monoclonal antibody, PPMS primary progressive multiple sclerosis, RMS relapsing forms of multiple sclerosis, S1P sphingosine 1-phosphate, SC subcutaneous injection, US United States
aHistorically, mitoxantrone was used as an induction agent, but its use as a multiple sclerosis treatment has decreased in recent years due to its toxicity and the introduction of newer, better-tolerated therapies
bClinically isolated syndrome, relapsing–remitting disease, and active secondary progressive disease
cSecondary progressive, progressive relapsing, or worsening relapsing–remitting disease
dRelapsing–remitting disease and active secondary progressive disease
Although exhaustive head-to-head studies have not been performed, data in pivotal phase III randomized controlled trials support that a subset of approved medications (including ofatumumab, ocrelizumab, natalizumab, alemtuzumab, and cladribine) are superior at preventing MS relapses, new MRI activity, and disease progression compared with either active comparators of lower efficacy (Supplementary Table 1, see electronic supplementary material [ESM]) [43–48] or placebo (Supplementary Table 2, see ESM) [25, 49–55]. Although not currently approved for MS, recent findings from a rater-blinded, phase III, multi-center Swedish study (RIFUND-MS) also support rituximab having better efficacy (reduced number of relapses) compared with dimethyl fumarate in RRMS [56]. Based on these efficacy data, as well as a limited number of indirect cross-trial comparisons, observational studies, and clinical experience, we classify these DMTs as high-efficacy [57–63]. It is important to note that there is currently a lack of consensus on the classification of some DMTs as ‘high-efficacy’ versus ‘moderate’ or ‘low-efficacy;’ as such, DMT classifications vary between studies. In particular, the S1P receptor modulators fingolimod and siponimod, as well as cladribine, are classified as HETs in some studies and non-HETs in others [64–67] (ClinicalTrials.gov identifiers: NCT03500328, NCT03535298).
Treatment Strategy (Escalation vs Early HET)
There are different treatment strategies for using these MS medications: the escalation approach, and the early HET approach (either using induction therapy or continuous treatment with high-efficacy DMTs).
The escalation approach, which calls for starting newly diagnosed patients on a low/moderate-efficacy therapy (e.g., IFNs, glatiramer acetate), has historically been utilized in clinical practice. If signs of breakthrough disease emerge, treatment is escalated to a HET. However, with the increasing availability of high-efficacy DMTs, and accumulating evidence of a narrow therapeutic window for effective MS treatment, this treatment approach is being re-evaluated in light of alternative strategies, outlined below.
The early HET approach advocates for the first-line use of HETs in people with RRMS at the time of diagnosis, irrespective of their age or disability level. Using HETs in the early stages of MS aims to decrease the clinically apparent and subclinical inflammatory activity that drives neuronal and brain volume loss (brain atrophy), preserve brain tissue and neurological reserve, and maximize long-term brain health [20, 68]. This strategy is similar to early aggressive treatment approaches for other immune-mediated diseases, such as rheumatoid arthritis, which are known to improve long-term clinical outcomes [69]. The early HET approach encompasses both induction therapy and continuous treatment with high-efficacy DMTs.
Induction therapy, or immune reconstitution therapy, is a form of HET that aims to ‘reset’ the immune system to provide long-lasting treatment effects. It involves the pulsed administration of immunosuppressive therapies, followed by a prolonged drug-free period or maintenance therapy with a low/moderate-efficacy DMT [70–72]. This treatment approach tends to be reserved for individuals with aggressive MS and poor prognosis at baseline [73]. HETs that are utilized as induction therapy include alemtuzumab (intravenous treatment courses administered 12 months apart) and cladribine (2-yearly treatment courses administered orally).
Other HETs are used continuously, with ongoing, long-term treatment administered at regular dosing intervals; these DMTs include ofatumumab, ocrelizumab, natalizumab, and rituximab (used off-label) [37, 74, 75]. Ofatumumab is administered subcutaneously monthly (following three initial doses) [74], without need for dose adjustment based on individual patient characteristics [76]. Ocrelizumab is administered intravenously every 6 months (following two initial doses) [37], and natalizumab is administered intravenously every 4 weeks [75].
Early Intervention with HETs Improves Short-Term and Long-Term Clinical Outcomes
Data in pivotal phase III randomized controlled trials for HETs with active comparators of lower efficacy (as summarized in Supplementary Table 1, see ESM) show that ofatumumab (ASCLEPIOS; vs teriflunomide), ocrelizumab (OPERA; vs IFNβ-1a), and alemtuzumab (CARE-MS; vs IFNβ-1a) reduced annualized relapse rates (ARR), disability worsening, new/enlarged MRI lesions, and brain volume loss in patients with RMS versus their respective active comparators [44–46, 48]. In the ASCLEPIOS and OPERA trials, a higher proportion of patients achieved NEDA-3 with ofatumumab (vs teriflunomide) and ocrelizumab (vs IFNβ-1a), respectively [44, 47]. These data offer a direct comparison of efficacy between HETs and lower-efficacy therapies. Placebo-controlled phase III trials of natalizumab and cladribine are summarized in Supplementary Table 2 (see ESM).
Long-term open-label extension (OLE) studies of these randomized clinical trials (Supplementary Table 3, see ESM), in which patients originally randomized to the lower-efficacy comparator therapy are switched to the HET after completion of the randomized treatment period, enable limited comparative evaluation of HET treatment effects delayed by up to 2 years following earlier lower-efficacy therapy. In the OLE studies for alemtuzumab and ocrelizumab, the patients switching from the lower-efficacy IFNβ-1a during the OLE phase responded to the HET similarly to those continuing on HET in terms of ARR and MRI disease activity. However, patients switching from IFNβ-1a had persistently higher disability levels, and exhibited more whole-brain volume loss, than those who had initiated earlier HET treatment [77, 78]. These findings highlight the clinical benefits of early use of HETs (vs delayed intervention) in RMS to optimize short-term and long-term outcomes. Efficacy data up to 4 years for the ongoing OLE study for ofatumumab are forthcoming in 2022 and will explore similar long-term efficacy outcomes versus patients on teriflunomide during the randomized treatment period (NCT03650114).
Findings from real-world studies suggest clinical benefits associated with HETs as first-line therapy in treatment-naïve patients, compared with first-line treatment with lower-efficacy therapies. A nationwide study in Denmark (n = 388) found that treatment-naïve patients with RMS receiving HET (defined as natalizumab, fingolimod, or alemtuzumab) as their first MS treatment had a lower probability of first relapse and of 6-month confirmed EDSS score worsening at 4 years of follow-up compared with a matched sample starting on a low/moderate-efficacy DMT (defined as IFNβ, teriflunomide, dimethyl fumarate, or glatiramer acetate) [66]. Another study in the United Kingdom (n = 592) found that patients with MS initially started on HETs (defined as alemtuzumab or natalizumab) had lower EDSS scores after 5 years of follow-up compared with those whose first treatment was a low/moderate-efficacy DMT (defined as IFNs, glatiramer acetate, dimethyl fumarate, fingolimod, or teriflunomide) [64]. A study in Italy (n = 2702) found increasing differences in disability trajectory over up to 10 years of follow-up between treatment-naïve patients who received a HET (defined as fingolimod, natalizumab, mitoxantrone, or cladribine) as their first treatment within 13 months of disease onset compared with their propensity score matched counterparts who received the HET after ≥ 1 year of low/moderate-efficacy treatment (glatiramer acetate, IFNs, or azathioprine) [79].
Studies assessing the timing of initiating treatment with HETs along the MS disease spectrum also generally support the notion of ‘earlier is better.’ An analysis of data from the MSBase registry (n = 308) and the Swedish MS registry (n = 236) found less disability after 6–10 years in patients who started HET (defined as rituximab, mitoxantrone, alemtuzumab, or natalizumab) within 2 years of disease onset compared with those who started 4–6 years after disease onset [80]. A systematic review evaluating the effect of HETs (defined as fingolimod, natalizumab, or alemtuzumab) at different stages of MS found that early HET initiation more potently suppressed relapse activity compared with delayed initiation; whether early versus delayed treatment initiation was associated with improved disability and MRI outcomes was inconclusive [65]. Another study found that initial treatment with fingolimod, alemtuzumab, or natalizumab was associated with a lower risk of conversion to SPMS versus initial treatment with glatiramer acetate or IFNβ-1a, and probability of conversion was lower when treatment was started within 5 years of disease onset versus after 5 years [81]. Of note, the real-world studies/analyses assessing long-term clinical outcomes described above include few/no patients on ofatumumab, ocrelizumab, and cladribine due to timings of study publications (2017–2021) and regulatory approvals (2017–2020; Table 1). It is important to note that in real-world evidence studies, assignment to HET or a lower-efficacy treatment is based on individual patient characteristics such as disease activity, rather than random assignment as in randomized controlled trials [64, 79]. While this limits direct comparisons of early HET versus an escalation strategy, these studies add support to the use of HET in the first-line setting, as patients with more active disease who were treated with HET experienced better outcomes than those with less active disease who were not treated with HET. Nevertheless, prospective head-to-head studies of first-line HET versus treatment escalation are required; the ongoing DELIVER-MS (NCT03535298) and TREAT-MS (NCT03500328) trials are directly comparing early intensive versus escalation approaches in a randomized pragmatic design to ascertain if systemic application of early HET improves the prognosis for patients with RRMS [82–84].
Risks of Serious Adverse Events Associated with HETs
The improved efficacy outcomes with HETs might seem to mandate their use for all newly diagnosed patients with MS. When practitioners consider HETs, their main concerns relate to potential adverse events, which are perceived to occur more frequently among patients treated with HETs. Safety data in pivotal phase III trials for HETs with active comparators of lower efficacy offer a comparison of serious adverse event (SAE) rates between therapies (Supplementary Table 1, see ESM) [43, 45, 46, 48], while extension studies [77, 78, 85–87], pooled analyses, and post-marketing surveillance can provide additional information on rare, delayed, or cumulative SAEs as these arise over time. SAEs associated with HETs vary by class of therapy and may include increased risk of hematologic abnormalities, infections, malignancy, secondary autoimmunity, neurovascular events, and teratogenicity.
Hematologic Abnormalities
Given the immunomodulatory nature of MS DMTs, treatment may be associated with shifts in circulating immune cells. Treatment with anti-CD20 monoclonal antibodies, which results in near-complete depletion of circulating B cells, could lead to a decline in serum immunoglobulin (Ig) levels (hypogammaglobulinemia) over time. In clinical trials of rituximab and ocrelizumab, reductions in IgM, IgG, and IgA were observed, with IgM reductions being most pronounced [39, 48, 77, 88, 89]. A pooled analysis of ocrelizumab clinical studies in RMS and PPMS patient populations and their OLEs (up to ~ 7 years of ocrelizumab exposure) have shown an association between decreased levels of IgG (levels less than the lower limit of normal) and increased rates of serious infections [90]. In a pooled analysis of the ocrelizumab OPERA study population (including 1174 patients who entered the OLE study and completed 5-year follow-up), the proportion of patients with decrease below the lower limit of normal at baseline and at year 5 were 0.5% and 29.5% for IgM, 0.5% and 5.4% for IgG, and 1.2% and 5.1% for IgA [77]. In a pooled analysis of ofatumumab clinical trials and OLE study (including 1969 patients with up to 3.5 years of follow-up), the proportion of patients who were below the lower limit of normal at any time post-baseline was 23.1% for IgM and 1.5% for IgG; no association was observed between decreased Ig levels and risk of serious infections with ofatumumab [91].
Alemtuzumab treatment may also lead to dose-dependent reductions of IgG, IgM, and IgA levels in serum and cerebrospinal fluid at 12 and 24 months following two courses, with further reductions in IgG in patients with persistent or returning disease activity treated with a third course of alemtuzumab [92]. Reductions in lymphocyte count (lymphopenia) have been observed with alemtuzumab and cladribine treatment. In MS clinical trials of alemtuzumab, nearly all (99.9%) alemtuzumab-treated patients experienced lymphopenia. The lowest lymphocyte counts occurred by ~ 1 month after each treatment course, and total lymphocyte counts increased to reach the lower limit of normal in ~ 40% of patients by 6 months after each alemtuzumab treatment course and in ~ 80% of patients by 12 months [42, 93]. In clinical studies of cladribine, 87% of cladribine-treated patients experienced lymphopenia, and reductions in lymphocyte count were dose dependent. The lowest absolute lymphocyte counts occurred ~ 2–3 months after the start of each treatment course and were lower with each subsequent course [41]; hypogammaglobulinemia has not been described for patients with MS taking cladribine [94]. Cases of severe (including fatal) neutropenia (low neutrophil counts) within 2 months of alemtuzumab infusion, and mild to moderate decreases in platelet counts (starting at the time of alemtuzumab infusion and often resolving without treatment), have also been reported [42].
It is unclear if anti-CD20s constitute induction therapy, and ongoing studies are addressing this [95, 96]. However, animal models demonstrate that B-cell–depleting anti-CD20 monoclonal antibodies do not achieve total depletion of tissue-resident B cells [97]. Moreover, upon treatment cessation, B cells reappear, first in the spleen and bone marrow, followed by reappearance in the blood; this reconstituted pool contains an elevated frequency of differentiated myelin-reactive B cells. These findings indicate that pathogenic B cells may persist following anti-CD20 treatment with rituximab or ocrelizumab, contributing to the recovering B-cell pool [97].
Despite the observations upon treatment cessation described above, no well-controlled data have yet shown a consistent increase of disease activity, or rebound activity, following discontinuation of anti-CD20 therapies or most HETs [37, 41, 42, 74, 75]. Rebound activity has been reported following discontinuation of sequestering therapies, such as fingolimod [98–100] and natalizumab [101–103]. However, rapid switch to an anti-CD20 treatment, such as ocrelizumab, may reduce this rebound risk [104].
Risk of Infections
Progressive Multifocal Leukoencephalopathy
Progressive multifocal leukoencephalopathy (PML), an opportunistic viral brain infection caused by the John Cunningham virus (JCV), can lead to death or severe disability [105]. Among the approved MS DMTs, PML incidence is highest with natalizumab [106]. Due to its boxed warning, natalizumab is available in the United States only through a restricted distribution program [75]. However, there is consensus in the United States that natalizumab can be used safely in patients who are anti-JCV antibody negative, as long as they are monitored for conversion to anti-JCV antibody positivity at least every 6 months. Estimated incidence of PML in the United States based on post-marketing data from ~ 100,000 natalizumab-exposed patients indicate a risk estimate of 1/10,000 for those who are anti-JCV antibody negative. In anti-JCV antibody-positive patients without prior immunosuppressant use, PML risk is estimated to be < 1/1000 in the first 24 months of natalizumab exposure, increasing to up to 4/1000 by 72 months of exposure [75], with further stratification of PML risk correlated to anti-JCV antibody serum concentrations [107]. Corresponding risk estimates for anti-JCV antibody-positive patients with prior immunosuppressant use are 1/1000 and 7/1000 [75]. Of note, the validated anti-JCV antibody test used to confirm the presence of JCV-specific antibodies has an analytical false-negative rate of 3% [75]. Extended interval dosing (6–8 weeks) instead of the approved standard interval dosing (every 4 weeks) has been suggested to reduce PML risk with natalizumab [108–110]; recent data showed an 88% reduction in PML risk with a ~ 6-week dosing schedule [111]. A recent phase IIIb trial evaluating 6-week versus 4-week interval dosing of natalizumab in patients with RRMS reported one case of PML with the 6-week interval dosing, but otherwise safety profiles were similar between the dosing regimens; a small but not significant reduction in efficacy was observed with the 6-week interval dosing regimen [112].
PML cases are rare in patients with MS following treatment with other HETs. In patients with MS treated with rituximab or ocrelizumab that developed PML, almost all cases were related to prior use of other DMTs such as natalizumab [113, 114]. However, the first case of PML occurring with 2-year ocrelizumab monotherapy in a 78-year-old man with progressive MS without prior immunomodulatory DMT use, who had exhibited intermittent mild (grade 1) lymphopenia prior to ocrelizumab initiation, was reported in 2021 [115]. The prescribing information for ocrelizumab has now been updated (August 2022) to include a warning for PML, based on the cases reported in patients treated with ocrelizumab, without previous natalizumab treatment, in the post-marketing setting [37]. There have been no confirmed cases of PML to date in patients with MS using subcutaneous ofatumumab [74] or cladribine [41]. One case of PML occurred in an alemtuzumab-treated patient with MS without prior exposure to natalizumab [116].
COVID-19
Given the ongoing COVID-19 pandemic, the potential association between MS, SARS-CoV-2 infection risk/severity, and DMT use has been a particularly highly debated topic of interest since early 2020. The current evidence suggests that having MS does not increase risk of SARS-CoV-2 infection or worsen clinical outcomes compared with those without MS [117]. However, risk factors including progressive MS, older age, higher levels of disability, obesity, diabetes, and cardiac and pulmonary comorbidities increase risk for more severe outcomes of COVID-19 for those who become infected [117, 118]. Additionally, given the immunomodulatory nature of many of the DMTs, some MS medications may increase the risk of a more severe COVID-19 disease course.
Current clinical evidence suggests that natalizumab, dimethyl fumarate, diroximel fumarate, teriflunomide, fingolimod, ozanimod, siponimod, IFNs, and glatiramer acetate do not increase risk of more severe COVID-19 symptoms [117–119]. An analysis of a global dataset of 2340 patients with MS (of whom 657 had suspected COVID-19 and 1683 had confirmed COVID-19) suggested a higher risk of more severe COVID-19 linked to rituximab (increased risk of hospitalization, intensive care unit [ICU] admission, and need for artificial ventilation) and ocrelizumab (increased risk of hospitalization and ICU admission) [120]. In a recent analysis of data from the ofatumumab ALITHIOS study (1703 people with MS, of whom 24 had suspected COVID-19 and 115 had confirmed COVID-19) and post-marketing surveillance (2 suspected and 26 confirmed COVID-19), COVID-19 incidence and severity in ofatumumab-treated patients with RMS were suggested to be comparable with the general population [121] (reference from congress proceedings). Considering the increased risk of severe COVID-19 associated with other anti-CD20 monoclonal antibodies, additional data are needed to further clarify COVID-19 risk on a larger number of ofatumumab-treated patients. Additional data are also needed for alemtuzumab and cladribine to assess their safety during the COVID-19 pandemic; however, initial reports do not suggest increased risk of severe COVID-19 with either treatment [122, 123]. The National Multiple Sclerosis Society recommends assessing use of anti-CD20 therapies, alemtuzumab, and cladribine during the pandemic, to balance potential COVID-19 risks and the consequences of delaying treatment with these HETs, including unintended outcomes such as worsening disability and increased relapse rate, MRI lesions, and brain atrophy, which will persist long after the pandemic [117, 118].
Due to the aforementioned shifts in circulating immune cells, patients using MS DMTs may have attenuated immune responses to vaccines. Given the direct effect of B-cell–depleting anti-CD20 monoclonal antibodies on humoral immunity, the extent or ability of patients with MS on these DMTs to mount a humoral immune response to COVID-19 vaccines are of particular concern. Studies suggest that rituximab- or ocrelizumab-treated MS patients generate reduced antibody responses to mRNA COVID-19 vaccines, but are able to mount SARS-CoV-2–specific T-cell responses that are comparable with responses in healthy individuals [124–128]. Data on timing of vaccination with ofatumumab treatment, which is currently limited and needs further confirmation, suggest that patients receiving ofatumumab treatment may be able to mount a stronger humoral response to the COVID-19 vaccine compared with those receiving ocrelizumab or rituximab [129]. Patients receiving natalizumab, alemtuzumab, or cladribine mounted full antibody responses to the mRNA COVID-19 vaccines that were comparable with those on lower-efficacy DMTs and no DMTs [128]. Recently issued COVID-19 vaccine guidance from the National Multiple Sclerosis Society encourages the vaccination of patients with MS and suggests that the timing of vaccination could be coordinated with that of DMT dosing [130, 131].
Malignancy
The immunosuppressive nature of MS DMTs may increase the risk of cancer in patients with MS [132]. Cladribine and alemtuzumab have boxed warnings that these medications may increase risk of malignancy, and cladribine is contraindicated in patients with current malignancies [41, 42]; ocrelizumab has a warning that it may increase risk of malignancy, including breast cancer [37]. A meta-analysis of 11 phase III clinical trials of cladribine and other DMTs (dimethyl fumarate, fingolimod, teriflunomide, natalizumab, alemtuzumab, glatiramer acetate) suggested that cancer rates with cladribine treatment were comparable with the other studied DMTs in patients with RMS [133]. Additionally, an integrated safety analysis for cladribine, including 1564 patients with RMS from the three phase III studies (CLARITY, CLARITY Extension, ORACLE-MS) and the completed PREMIERE safety registry, found that malignancy incidence with cladribine was not statistically different from placebo (observation-adjusted incidence rates per 100 patient-years of 0.26 and 0.12, respectively) and the matched GLOBOCAN reference population (standardized incidence ratio, 0.88; 95% CI 0.44–1.69) [134]. For alemtuzumab, a 5-year follow up of the CARE-MS I and II studies found that 10 of 811 alemtuzumab-treated patients developed malignancies over 5 years [135, 136]; malignancy rates with alemtuzumab from the CARE-MS extension trial (exposure up to 6 years) was ≤ 0.4% per year [78]. Malignancy rates in patients treated with rituximab or ocrelizumab do not indicate an increased risk compared with matched reference populations [90, 137]. There was no indication of an excess of breast cancer incidence in women treated with ocrelizumab in the US real-world setting compared with the US general population [90]. Based on the ofatumumab phase III ASCLEPIOS studies, five neoplasms (0.5%) occurred in the ofatumumab group (n = 946) versus four (0.4%) in the teriflunomide group (n = 936), with no indication of an increased risk of malignancy [43]. A 10-year interim report of a real-world study of 6148 natalizumab-treated patients with RRMS indicated malignancy incidence rates of 1.1% (n = 66); of these, breast cancer was the most common neoplasm (n = 19; 86.7 per 100,000 patient-years), which is in line with the matched SEER and GLOBOCAN reference populations [138].
Other SAEs: Secondary Autoimmunity, Neurovascular Events, Teratogenicity
Alemtuzumab treatment can result in an increased risk of serious (sometimes fatal) autoimmune conditions and stroke. Alemtuzumab-treated patients in randomized trials and open-label extension studies experienced thyroid disorders (36.8%), immune thrombocytopenia (2%), glomerular nephropathies (0.3%), vitiligo (0.3%), autoimmune hemolytic anemia (0.3%), autoimmune pancytopenia (0.2%), undifferentiated connective tissue disorders (0.2%), type 1 diabetes mellitus (0.2%), rheumatoid arthritis (0.1%), retinal pigment epitheliopathy (0.1%), and acquired hemophilia A (0.1%) [42]. A recent safety analysis of the alemtuzumab clinical trial program found that post-alemtuzumab treatment autoimmune AEs were similar between patients with preexisting non-MS autoimmunity (35.4%) and those without (35.3%), and these AEs were serious in 8.8% and 9.1% of cases, respectively [139]. Ischemic and hemorrhagic stroke or arterial dissection occurring shortly after alemtuzumab treatment were reported in 13 cases worldwide since FDA approval in 2014 [140]. Cladribine is contraindicated in pregnant women due to evidence of malformations and embryolethality in animals [41]. Pregnancy outcomes in an integrated safety analysis of the cladribine clinical development program, the results of which are limited by the small number of pregnancies that occurred (70 direct pregnancies and 9 partner pregnancies), were generally consistent with epidemiological data for women with MS or the general population [141].
Expert Perspectives and Future Outlook
The MS treatment landscape has evolved in recent years with the increasing availability of high-efficacy DMTs with good safety profiles, including anti-CD20 monoclonal antibodies with fewer safety monitoring requirements compared with other HETs. Several classes of MS therapies are currently in clinical development (their relative clinical efficacy yet to be determined), including Bruton’s tyrosine kinase inhibitors [142], and Epstein-Barr virus–specific T-cell therapy [143, 144]. Autologous hematopoietic stem cell transplantation (AHSCT) has been of increasing interest in recent years as an MS treatment option. Several clinical trials and meta-analyses have reported that, in some patients with relapsing disease, AHSCT was able to induce long-term disease remission, delay disease progression compared with DMTs (including HETs), and even halt all detectable CNS inflammatory activity, although methodological differences within and across the existing studies limited their interpretability [145–148]. Ongoing randomized clinical trials (e.g., BEAT-MS [NCT04047628] and STAR-MS [ISRCTN registry identifier: ISRCTN88667898]) are comparing AHSCT versus HETs (cladribine, natalizumab, alemtuzumab, ocrelizumab, rituximab, or ofatumumab) and will help better define the optimal utilization of AHSCT. At present, AHSCT is an option, albeit limited in access outside of clinical trials, for patients with aggressive RMS, or those who exhibit high clinical or MRI activity despite HET treatment [149–151]. While AHSCT may become an important component of the MS armamentarium, it remains to be determined whether it would be suitable for treatment-naïve patients.
Clinical evidence of a narrow therapeutic window for effective MS treatment in the early stages of disease is emerging. Currently, it is not possible for clinicians to accurately predict prognosis and treatment response at disease onset at an individual patient level [27], and subclinical disease processes may occur despite treatment. MS relapses, and the formation of new MRI lesions in the CNS indicating inflammatory activity, are well established contributors to neurological disability and correlate with prognosis in the short term [19, 29]. While these parameters meaningfully contribute to long-term disability [152], disability worsening may also occur in the absence of relapses or new brain lesions [19]. The inability to predict poor clinical outcome at an individual patient level, coupled with the potential for disability progression independent of relapse activity, support the use of HETs as first-line treatment for most newly diagnosed patients with RMS. In addition, the labels of some HETs, such as ocrelizumab and natalizumab, have been expanded from RMS to encompass any ‘active’ form of MS, including CIS and active SPMS [37, 75]. However, safety concerns around HET use, as well as the variability of MS severity, may support arguments against their use in all such patients. Additional data on long-term safety of these DMTs, as well as an increased understanding of predictors of poor clinical outcomes, would help clinicians and patients to decide whether treatment with HETs is right for them. Ongoing clinical trials assessing early RRMS treatment with HETs compared with other treatment approaches include DELIVER-MS (study completion in 2026) [67] and TREAT-MS (NCT03500328; estimated study completion in 2025). Based on the need for efficacious treatment in the early stages of MS disease, we believe HETs are appropriate for most patients with RMS as a first-line treatment option. However, a shared decision-making approach should occur between the patient and their care teams, including comprehensive discussions on available treatment options as well as the benefits and risks of early HET use, to ensure that an informed decision is made as a team on the appropriate treatment.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Medical writing support, which included development of the first draft under author guidance, was provided by Grace Jeong, PhD, of Alphabet Health (New York, NY, USA) and was funded by Novartis Pharmaceuticals Corporation.
Declarations
Funding
Medical writing support and open access publication was funded by Novartis Pharmaceuticals Corporation.
Conflicts of interest/Competing interests
Léorah Freeman has received consultancy fees from Celgene/Bristol Myers Squibb, EMD Serono, Genentech, Novartis, and TG Therapeutics; and has received program sponsorship from EMD Serono. She has grant support from EMD Serono, Genentech, NIH/NINDS, and PCORI. Erin E. Longbrake has received honoraria from Bristol Myers Squibb, Genentech, Janssen, NGM Bio, and TG Therapeutics. She has research support from Genentech, NIH K23NS107624, Race to Erase MS, and
UL1 TR001863. Patricia K. Coyle received consultancy fees from Accordant, Alexion, Bayer, Biogen, Bristol Myers Squibb, Celgene, EMD Serono, Genentech/Roche, GlaxoSmithKline, Horizon Therapeutics, Janssen, Mylan, Novartis, Sanofi Genzyme, TG Therapeutics, and Viela Bio; and has received research grants from Actelion, Alkermes, Corrona, Genentech/Roche, MedDay, NINDS, and Novartis. Barry Hendin has received research support and advisory and speaking honoraria from Alexion, Biogen, EMD Serono, Genentech, Genzyme, and Novartis. Timothy Vollmer has received compensation for lectures and consultancy from Biogen, Celgene, EMD Serono, Genentech/Roche, Novartis, and Siranax; and has received research support from Anokion, Biogen, Celgene, F. Hoffmann-La Roche Ltd., Genentech, GW Pharma, Rocky Mountain Multiple Sclerosis Center, and TG Therapeutics.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Authors’ contributions
All authors discussed and agreed on the concept for this article. Grace Jeong performed the literature search; Léorah Freeman, Erin E. Longbrake, Patricia K. Coyle, Barry Hendin, and Timothy Vollmer drafted and/or critically reviewed the work. All authors have read and approved the final submitted manuscript, and agree to be accountable for the work. This manuscript was developed in accordance with Good Publication Practice (GPP3) guidelines. Authors had full control of the content and made the final decision on all aspects of this publication.
References
- 1.Filippi M, Bar-Or A, Piehl F, Preziosa P, Solari A, Vukusic S, et al. Multiple sclerosis. Nat Rev Dis Primers. 2018;4:43. doi: 10.1038/s41572-018-0041-4. [DOI] [PubMed] [Google Scholar]
- 2.Reich DS, Lucchinetti CF, Calabresi PA. Multiple sclerosis. N Engl J Med. 2018;378:169–180. doi: 10.1056/NEJMra1401483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Multiple Sclerosis Society. Medications. https://www.nationalmssociety.org/Treating-MS/Medications. Accessed 1 Feb 2022.
- 4.National Multiple Sclerosis Society. Disease-modifying therapies for MS. https://www.nationalmssociety.org/NationalMSSociety/media/MSNationalFiles/Brochures/Brochure-The-MS-Disease-Modifying-Medications.pdf. Accessed 1 Feb 2022.
- 5.European Multiple Sclerosis Platform. MS treatments. https://emsp.org/about-ms/ms-treatments/. Accessed 29 July 2022.
- 6.European Medicines Agency. Vumerity [summary of product characteristics]. https://www.ema.europa.eu/en/documents/product-information/vumerity-epar-product-information_en.pdf. Accessed 29 July 2022.
- 7.Montalban X, Gold R, Thompson AJ, Otero-Romero S, Amato MP, Chandraratna D, et al. ECTRIMS/EAN guideline on the pharmacological treatment of people with multiple sclerosis. Mult Scler. 2018;24:96–120. doi: 10.1177/1352458517751049. [DOI] [PubMed] [Google Scholar]
- 8.Rae-Grant A, Day GS, Marrie RA, Rabinstein A, Cree BAC, Gronseth GS, et al. Comprehensive systematic review summary: disease-modifying therapies for adults with multiple sclerosis: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2018;90:789–800. doi: 10.1212/WNL.0000000000005345. [DOI] [PubMed] [Google Scholar]
- 9.Marziniak M, Ghorab K, Kozubski W, Pfleger C, Sousa L, Vernon K, et al. Variations in multiple sclerosis practice within Europe—is it time for a new treatment guideline? Mult Scler Relat Disord. 2016;8:35–44. doi: 10.1016/j.msard.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) advisory committee on clinical trials of new agents in multiple sclerosis. Neurology. 1996;46:907–911. doi: 10.1212/WNL.46.4.907. [DOI] [PubMed] [Google Scholar]
- 11.Lublin FD, Reingold SC, Cohen JA, Cutter GR, Sørensen PS, Thompson AJ, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014;83:278–286. doi: 10.1212/WNL.0000000000000560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Multiple Sclerosis Society. Types of MS. https://www.nationalmssociety.org/What-is-MS/Types-of-MS. Accessed 1 Feb 2022.
- 13.Bjornevik K, Munger KL, Cortese M, Barro C, Healy BC, Niebuhr DW, et al. Serum neurofilament light chain levels in patients with presymptomatic multiple sclerosis. JAMA Neurol. 2020;77:58–64. doi: 10.1001/jamaneurol.2019.3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Azevedo CJ, Overton E, Khadka S, Buckley J, Liu S, Sampat M, et al. Early CNS neurodegeneration in radiologically isolated syndrome. Neurol Neuroimmunol Neuroinflamm. 2015;2:e102. doi: 10.1212/NXI.0000000000000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.George IC, El Mendili MM, Inglese M, Azevedo CJ, Kantarci O, Lebrun C, et al. Cerebellar volume loss in radiologically isolated syndrome. Mult Scler. 2021;27:130–133. doi: 10.1177/1352458519887346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rojas JI, Patrucco L, Míguez J, Besada C, Cristiano E. Brain atrophy in radiologically isolated syndromes. J Neuroimaging. 2015;25:68–71. doi: 10.1111/jon.12182. [DOI] [PubMed] [Google Scholar]
- 17.De Stefano N, Giorgio A, Battaglini M, Rovaris M, Sormani MP, Barkhof F, et al. Assessing brain atrophy rates in a large population of untreated multiple sclerosis subtypes. Neurology. 2010;74:1868–1876. doi: 10.1212/WNL.0b013e3181e24136. [DOI] [PubMed] [Google Scholar]
- 18.Paolillo A, Piattella MC, Pantano P, Di Legge S, Caramia F, Russo P, et al. The relationship between inflammation and atrophy in clinically isolated syndromes suggestive of multiple sclerosis. J Neurol. 2004;251:432–439. doi: 10.1007/s00415-004-0349-8. [DOI] [PubMed] [Google Scholar]
- 19.Cree BAC, Hollenbach JA, Bove R, Kirkish G, Sacco S, Caverzasi E, University of California San Francisco MS-EPIC Team et al. Silent progression in disease activity-free relapsing multiple sclerosis. Ann Neurol. 2019;85:653–666. doi: 10.1002/ana.25463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vollmer TL, Nair KV, Williams I, Alvarez E. Multiple sclerosis phenotypes as a continuum: the role of neurological reserve. Neurol Clin Pract. 2021;11:342–351. doi: 10.1212/CPJ.0000000000001045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oh J, Vidal-Jordana A, Montalban X. Multiple sclerosis: clinical aspects. Curr Opin Neurol. 2018;31:752–759. doi: 10.1097/WCO.0000000000000622. [DOI] [PubMed] [Google Scholar]
- 22.Kister I, Bacon T, Cutter GR. How multiple sclerosis symptoms vary by age, sex, and race/ethnicity. Neurol Clin Pract. 2021;11:335–341. doi: 10.1212/CPJ.0000000000001105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prosperini L, Capobianco M, Giannì C. Identifying responders and nonresponders to interferon therapy in multiple sclerosis. Degener Neurol Neuromuscul Dis. 2014;4:75–85. doi: 10.2147/DNND.S42734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dominguez-Mozo MI, Perez-Perez S, Villar LM, Oliver-Martos B, Villarrubia N, Matesanz F, et al. Predictive factors and early biomarkers of response in multiple sclerosis patients treated with natalizumab. Sci Rep. 2020;10:14244. doi: 10.1038/s41598-020-71283-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giovannoni G, Comi G, Cook S, Rammohan K, Rieckmann P, Soelberg Sørensen P, CLARITY Study Group et al. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med. 2010;362:416–426. doi: 10.1056/NEJMoa0902533. [DOI] [PubMed] [Google Scholar]
- 26.Naismith RT. Multiple sclerosis therapeutic strategies: start safe and effective, reassess early, and escalate if necessary. Neurol Clin Pract. 2011;1:69–72. doi: 10.1212/CPJ.0b013e31823cc2b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bovis F, Carmisciano L, Signori A, Pardini M, Steinerman JR, Li T, et al. Defining responders to therapies by a statistical modeling approach applied to randomized clinical trial data. BMC Med. 2019;17:113. doi: 10.1186/s12916-019-1345-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith AL, Cohen JA, Hua LH. Therapeutic targets for multiple sclerosis: current treatment goals and future directions. Neurotherapeutics. 2017;14:952–960. doi: 10.1007/s13311-017-0548-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giovannoni G. Disease-modifying treatments for early and advanced multiple sclerosis: a new treatment paradigm. Curr Opin Neurol. 2018;31:233–243. doi: 10.1097/WCO.0000000000000561. [DOI] [PubMed] [Google Scholar]
- 30.Colligan E, Metzler A, Tiryaki E. Shared decision-making in multiple sclerosis. Mult Scler. 2017;23:185–190. doi: 10.1177/1352458516671204. [DOI] [PubMed] [Google Scholar]
- 31.Col NF, Solomon AJ, Springmann V, Ionete C, Alvarez E, Tierman B, et al. Evaluation of a novel preference assessment tool for patients with multiple sclerosis. Int J MS Care. 2018;20:260–267. doi: 10.7224/1537-2073.2017-021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davis BE, Lakin L, Binns CC, Currie KM, Rensel MR. Patient and provider insights into the impact of multiple sclerosis on mental health: a narrative review. Neurol Ther. 2021;10:99–119. doi: 10.1007/s40120-021-00240-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lakin L, Davis BE, Binns CC, Currie KM, Rensel MR. Comprehensive approach to management of multiple sclerosis: addressing invisible symptoms—a narrative review. Neurol Ther. 2021;10:75–98. doi: 10.1007/s40120-021-00239-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.European Medicines Agency. Assessment report: Plegridy. https://www.ema.europa.eu/en/documents/variation-report/plegridy-h-c-2827-x-56-epar-assessment-report-variation_en.pdf. Accessed 6 Aug 2022.
- 35.European Medicines Agency. Dimethyl fumarate Neuraxpharm (dimethyl fumarate): an overview of Dimethyl fumarate Neuraxpharm and why it is authorised in the EU. https://www.ema.europa.eu/en/documents/overview/dimethyl-fumarate-neuraxpharm-epar-medicine-overview_en.pdf. Accessed 6 Aug 2022.
- 36.National Multiple Sclerosis Society. FDA approves intramuscular Plegridy for relapsing MS. https://www.nationalmssociety.org/About-the-Society/News/FDA-Approves-Intramuscular-Plegridy-for-Relapsing. Accessed 6 Aug 2022.
- 37.Genentech, Inc. OCREVUS® (ocrelizumab) [package insert]. https://www.gene.com/download/pdf/ocrevus_prescribing.pdf. Accessed 31 Aug 2022.
- 38.Novartis Pharmaceuticals Corporation. ARZERRA® (ofatumumab) [package insert]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/125326s062lbl.pdf. Accessed 3 June 2021.
- 39.Genentech, Inc. RITUXAN® (rituximab) [package insert]. https://www.gene.com/download/pdf/rituxan_prescribing.pdf. Accessed 3 Feb 2022.
- 40.Sabatino JJ, Jr, Zamvil SS, Hauser SL. B-cell therapies in multiple sclerosis. Cold Spring Harb Perspect Med. 2019;9:a032037. doi: 10.1101/cshperspect.a032037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.EMD Serono. MAVENCLAD® (cladribine) [package insert]. https://www.mavenclad.com/content/dam/web/healthcare/neurology/united-states/pdfs/Prescribing%20Information.pdf. Accessed 1 Feb 2022.
- 42.Genzyme Corporation. LEMTRADA® (alemtuzumab) [package insert]. https://products.sanofi.us/lemtrada/lemtrada.pdf. Accessed 10 Feb 2022.
- 43.Hauser SL, Bar-Or A, Cohen JA, Comi G, Correale J, Coyle PK, ASCLEPIOS I and ASCLEPIOS II Trial Groups et al. Ofatumumab versus teriflunomide in multiple sclerosis. N Engl J Med. 2020;383:546–557. doi: 10.1056/NEJMoa1917246. [DOI] [PubMed] [Google Scholar]
- 44.Hauser SL, Bar-Or A, Cohen JA, Comi G, Correale J, Coyle PK, et al. Ofatumumab vs teriflunomide in relapsing multiple sclerosis: analysis of no evidence of disease activity (NEDA-3) from ASCLEPIOS I and II trials. Eur J Neurol. 2020;27:1289–1290. [Google Scholar]
- 45.Cohen JA, Coles AJ, Arnold DL, Confavreux C, Fox EJ, Hartung HP, CARE-MS I investigators et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet. 2012;380:1819–1828. doi: 10.1016/S0140-6736(12)61769-3. [DOI] [PubMed] [Google Scholar]
- 46.Coles AJ, Twyman CL, Arnold DL, Cohen JA, Confavreux C, Fox EJ, CARE-MS II investigators et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet. 2012;380:1829–1839. doi: 10.1016/S0140-6736(12)61768-1. [DOI] [PubMed] [Google Scholar]
- 47.Giovannoni G, Arnold DL, Bar-Or A, Havrdová E, Kappos L, Lublin F, et al. PO129 Neda analysis by epoch in the opera studies of ocrelizumab. J Neurol Neurosurg Psychiatry. 2017;88:A46. doi: 10.1136/jnnp-2017-ABN.159. [DOI] [Google Scholar]
- 48.Hauser SL, Bar-Or A, Comi G, Giovannoni G, Hartung HP, Hemmer B, OPERA I and OPERA II Clinical Investigators et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376:221–234. doi: 10.1056/NEJMoa1601277. [DOI] [PubMed] [Google Scholar]
- 49.Havrdova E, Galetta S, Hutchinson M, Stefoski D, Bates D, Polman CH, et al. Effect of natalizumab on clinical and radiological disease activity in multiple sclerosis: a retrospective analysis of the Natalizumab Safety and Efficacy in Relapsing-Remitting Multiple Sclerosis (AFFIRM) study. Lancet Neurol. 2009;8:254–260. doi: 10.1016/S1474-4422(09)70021-3. [DOI] [PubMed] [Google Scholar]
- 50.Polman CH, O'Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH, AFFIRM Investigators et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354:899–910. doi: 10.1056/NEJMoa044397. [DOI] [PubMed] [Google Scholar]
- 51.Miller DH, Soon D, Fernando KT, MacManus DG, Barker GJ, Yousry TA, AFFIRM Investigators et al. MRI outcomes in a placebo-controlled trial of natalizumab in relapsing MS. Neurology. 2007;68:1390–1401. doi: 10.1212/01.wnl.0000260064.77700.fd. [DOI] [PubMed] [Google Scholar]
- 52.Phillips JT, Giovannoni G, Lublin FD, O’Connor PW, Polman CH, Willoughby E, et al. Sustained improvement in Expanded Disability Status Scale as a new efficacy measure of neurological change in multiple sclerosis: treatment effects with natalizumab in patients with relapsing multiple sclerosis. Mult Scler. 2011;17:970–979. doi: 10.1177/1352458511399611. [DOI] [PubMed] [Google Scholar]
- 53.De Stefano N, Giorgio A, Battaglini M, De Leucio A, Hicking C, Dangond F, et al. Reduced brain atrophy rates are associated with lower risk of disability progression in patients with relapsing multiple sclerosis treated with cladribine tablets. Mult Scler. 2018;24:222–226. doi: 10.1177/1352458517690269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Giovannoni G, Cook S, Rammohan K, Rieckmann P, Sørensen PS, Vermersch P, et al. Sustained disease-activity-free status in patients with relapsing-remitting multiple sclerosis treated with cladribine tablets in the CLARITY study: a post-hoc and subgroup analysis. Lancet Neurol. 2011;10:329–337. doi: 10.1016/S1474-4422(11)70023-0. [DOI] [PubMed] [Google Scholar]
- 55.Leist TP, Comi G, Cree BA, Coyle PK, Freedman MS, Hartung H-P, oral cladribine for early MS (ORACLE MS) Study Group et al. Effect of oral cladribine on time to conversion to clinically definite multiple sclerosis in patients with a first demyelinating event (ORACLE MS): a phase 3 randomised trial. Lancet Neurol. 2014;13:257–267. doi: 10.1016/S1474-4422(14)70005-5. [DOI] [PubMed] [Google Scholar]
- 56.Svenningsson A, Frisell T, Burman J, Salzer J, Fink K, Hallberg S, et al. Safety and efficacy of rituximab versus dimethyl fumarate in patients with relapsing-remitting multiple sclerosis or clinically isolated syndrome in Sweden: a rater-blinded, phase 3, randomised controlled trial. Lancet Neurol. 2022;21:693–703. doi: 10.1016/S1474-4422(22)00209-5. [DOI] [PubMed] [Google Scholar]
- 57.Spelman T, Kalincik T, Jokubaitis V, Zhang A, Pellegrini F, Wiendl H, et al. Comparative efficacy of first-line natalizumab vs IFN-beta or glatiramer acetate in relapsing MS. Neurol Clin Pract. 2016;6:102–115. doi: 10.1212/CPJ.0000000000000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Spelman T, Kalincik T, Zhang A, Pellegrini F, Wiendl H, Kappos L, et al. Comparative efficacy of switching to natalizumab in active multiple sclerosis. Ann Clin Transl Neurol. 2015;2:373–387. doi: 10.1002/acn3.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Signori A, Sacca F, Lanzillo R, Maniscalco GT, Signoriello E, Repice AM, et al. Cladribine vs other drugs in MS: merging randomized trial with real-life data. Neurol Neuroimmunol Neuroinflamm. 2020;7:e878. doi: 10.1212/NXI.0000000000000878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alping P, Frisell T, Novakova L, Islam-Jakobsson P, Salzer J, Bjorck A, et al. Rituximab versus fingolimod after natalizumab in multiple sclerosis patients. Ann Neurol. 2016;79:950–958. doi: 10.1002/ana.24651. [DOI] [PubMed] [Google Scholar]
- 61.Granqvist M, Boremalm M, Poorghobad A, Svenningsson A, Salzer J, Frisell T, et al. Comparative effectiveness of rituximab and other initial treatment choices for multiple sclerosis. JAMA Neurol. 2018;75:320–327. doi: 10.1001/jamaneurol.2017.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kalincik T, Horakova D, Spelman T, Jokubaitis V, Trojano M, Lugaresi A, MSBase Study Group et al. Switch to natalizumab versus fingolimod in active relapsing-remitting multiple sclerosis. Ann Neurol. 2015;77:425–435. doi: 10.1002/ana.24339. [DOI] [PubMed] [Google Scholar]
- 63.Kalincik T, Brown JWL, Robertson N, Willis M, Scolding N, Rice CM, et al. Treatment effectiveness of alemtuzumab compared with natalizumab, fingolimod, and interferon beta in relapsing-remitting multiple sclerosis: a cohort study. Lancet Neurol. 2017;16:271–281. doi: 10.1016/S1474-4422(17)30007-8. [DOI] [PubMed] [Google Scholar]
- 64.Harding K, Williams O, Willis M, Hrastelj J, Rimmer A, Joseph F, et al. Clinical outcomes of escalation vs early intensive disease-modifying therapy in patients with multiple sclerosis. JAMA Neurol. 2019;76:536–541. doi: 10.1001/jamaneurol.2018.4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Merkel B, Butzkueven H, Traboulsee AL, Havrdova E, Kalincik T. Timing of high-efficacy therapy in relapsing-remitting multiple sclerosis: a systematic review. Autoimmun Rev. 2017;16:658–665. doi: 10.1016/j.autrev.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 66.Buron MD, Chalmer TA, Sellebjerg F, Barzinji I, Christensen JR, Christensen MK, et al. Initial high-efficacy disease-modifying therapy in multiple sclerosis: a nationwide cohort study. Neurology. 2020;95:e1041–e1051. doi: 10.1212/WNL.0000000000010135. [DOI] [PubMed] [Google Scholar]
- 67.Ontaneda D, Tallantyre EC, Raza PC, Planchon SM, Nakamura K, Miller D, et al. Determining the effectiveness of early intensive versus escalation approaches for the treatment of relapsing-remitting multiple sclerosis: the DELIVER-MS study protocol. Contemp Clin Trials. 2020;95:106009. doi: 10.1016/j.cct.2020.106009. [DOI] [PubMed] [Google Scholar]
- 68.Giovannoni G, Butzkueven H, Dhib-Jalbut S, Hobart J, Kobelt G, Pepper G, et al. Brain health: time matters in multiple sclerosis. Mult Scler Relat Disord. 2016;9:S5–48. doi: 10.1016/j.msard.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 69.Emery P, Duquenne L. It's never too soon to treat rheumatoid arthritis: finally, some supportive evidence. Lancet Rheumatol. 2020;2:e311–e313. doi: 10.1016/S2665-9913(20)30103-X. [DOI] [PubMed] [Google Scholar]
- 70.Coyle PK. Commentary: the multiple sclerosis controversy: is it escalation or induction high efficacy? Neurotherapeutics. 2020;17:971–972. doi: 10.1007/s13311-020-00869-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Prosperini L, Mancinelli CR, Solaro CM, Nociti V, Haggiag S, Cordioli C, et al. Induction versus escalation in multiple sclerosis: a 10-year real world study. Neurotherapeutics. 2020;17:994–1004. doi: 10.1007/s13311-020-00847-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.AlSharoqi IA, Aljumah M, Bohlega S, Boz C, Daif A, El-Koussa S, et al. Immune reconstitution therapy or continuous immunosuppression for the management of active relapsing-remitting multiple sclerosis patients? A narrative review. Neurol Ther. 2020;9:55–66. doi: 10.1007/s40120-020-00187-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fernández Ó. Is there a change of paradigm towards more effective treatment early in the course of apparent high-risk MS? Mult Scler Relat Disord. 2017;17:75–83. doi: 10.1016/j.msard.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 74.Novartis Pharmaceuticals Corporation. KESIMPTA® (ofatumumab) [package insert]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/125104s966lbl.pdf. Accessed 28 July 2022.
- 75.TYSABRI (natalizumab) [package insert].https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/125104s966lbl.pdf. Accessed 28 July 2022.
- 76.Yu H, Graham G, David OJ, Kahn JM, Savelieva M, Pigeolet E, et al. Population pharmacokinetic-B cell modeling for ofatumumab in patients with relapsing multiple sclerosis. CNS Drugs. 2022;36:283–300. doi: 10.1007/s40263-021-00895-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hauser SL, Kappos L, Arnold DL, Bar-Or A, Brochet B, Naismith RT, et al. Five years of ocrelizumab in relapsing multiple sclerosis: OPERA studies open-label extension. Neurology. 2020;95:e1854–e1867. doi: 10.1212/WNL.0000000000010376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Coles AJ, Arnold DL, Bass AD, Boster AL, Compston DAS, Fernández Ó, et al. Efficacy and safety of alemtuzumab over 6 years: final results of the 4-year CARE-MS extension trial. Ther Adv Neurol Disord. 2021;14:1756286420982134. doi: 10.1177/1756286420982134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Iaffaldano P, Lucisano G, Caputo F, Paolicelli D, Patti F, Zaffaroni M, Italian MS Register et al. Long-term disability trajectories in relapsing multiple sclerosis patients treated with early intensive or escalation treatment strategies. Ther Adv Neurol Disord. 2021;14:17562864211019574. doi: 10.1177/17562864211019574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.He A, Merkel B, Brown JWL, Zhovits Ryerson L, Kister I, Malpas CB, MSBase study group et al. Timing of high-efficacy therapy for multiple sclerosis: a retrospective observational cohort study. Lancet Neurol. 2020;19:307–316. doi: 10.1016/S1474-4422(20)30067-3. [DOI] [PubMed] [Google Scholar]
- 81.Brown JWL, Coles A, Horakova D, Havrdova E, Izquierdo G, Prat A, MSBase Study Group et al. Association of initial disease-modifying therapy with later conversion to secondary progressive multiple sclerosis. JAMA. 2019;321:175–187. doi: 10.1001/jama.2018.20588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.ClinicalTrials.gov. Determining the Effectiveness of earLy Intensive Versus Escalation Approaches for RRMS (DELIVER-MS). https://clinicaltrials.gov/ct2/show/NCT03535298. Accessed 11 Aug 2022.
- 83.Ziemssen T, Hoffmann F, Richter S, Engelmann U, White R. Alemtuzumab in a large real-life cohort: Interim baseline data of the TREAT-MS study. Front Neurol. 2021;12:620758. doi: 10.3389/fneur.2021.620758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ziemssen T, Engelmann U, Jahn S, Leptich A, Kern R, Hassoun L, et al. Rationale, design, and methods of a non-interventional study to establish safety, effectiveness, quality of life, cognition, health-related and work capacity data on alemtuzumab in multiple sclerosis patients in Germany (TREAT-MS) BMC Neurol. 2016;16:109. doi: 10.1186/s12883-016-0629-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hauser SL, Cross AH, Winthrop K, Wiendl H, Nicholas J, Meuth SG, et al. Safety experience with continued exposure to ofatumumab in patients with relapsing forms of multiple sclerosis for up to 3.5 years. Mult Scler. 2022;28:1576–1590. doi: 10.1177/13524585221079731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Giovannoni G, Soelberg Sorensen P, Cook S, Rammohan K, Rieckmann P, Comi G, et al. Safety and efficacy of cladribine tablets in patients with relapsing-remitting multiple sclerosis: results from the randomized extension trial of the CLARITY study. Mult Scler. 2018;24:1594–1604. doi: 10.1177/1352458517727603. [DOI] [PubMed] [Google Scholar]
- 87.Wolinsky JS, Arnold DL, Brochet B, Hartung H-P, Montalban X, Naismith RT, et al. Long-term follow-up from the ORATORIO trial of ocrelizumab for primary progressive multiple sclerosis: a post-hoc analysis from the ongoing open-label extension of the randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2020;19:998–1009. doi: 10.1016/S1474-4422(20)30342-2. [DOI] [PubMed] [Google Scholar]
- 88.Bar-Or A, Calabresi PA, Arnold D, Markowitz C, Shafer S, Kasper LH, et al. Rituximab in relapsing-remitting multiple sclerosis: a 72-week, open-label, phase I trial. Ann Neurol. 2008;63:395–400. doi: 10.1002/ana.21363. [DOI] [PubMed] [Google Scholar]
- 89.Hauser SL, Waubant E, Arnold DL, Vollmer T, Antel J, Fox RJ, HERMES Trial Group et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358:676–688. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- 90.Hauser SL, Kappos L, Montalban X, Craveiro L, Chognot C, Hughes R, et al. Safety of ocrelizumab in patients with relapsing and primary progressive multiple sclerosis. Neurology. 2021;97:e1546–e1559. doi: 10.1212/WNL.0000000000012700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wiendl H, de Seze J, Bar-Or A, Correale J, Cross AH, Kappos L, et al. Effect of ofatumumab on serum immunoglobulin levels and infection risk in patients with relapsing multiple sclerosis over 3.5 years. Mult Scler. 2021;27:P931. [Google Scholar]
- 92.Möhn N, Pfeuffer S, Ruck T, Gross CC, Skripuletz T, Klotz L, et al. Alemtuzumab therapy changes immunoglobulin levels in peripheral blood and CSF. Neurol Neuroimmunol Neuroinflamm. 2020;7:e654. doi: 10.1212/NXI.0000000000000654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li Z, Richards S, Surks HK, Jacobs A, Panzara MA. Clinical pharmacology of alemtuzumab, an anti-CD52 immunomodulator, in multiple sclerosis. Clin Exp Immunol. 2018;194:295–314. doi: 10.1111/cei.13208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Spiezia AL, Cerbone V, Molinari EA, Capasso N, Lanzillo R, Carotenuto A, et al. Changes in lymphocytes, neutrophils and immunoglobulins in year-1 cladribine treatment in multiple sclerosis. Mult Scler Relat Disord. 2022;57:103431. doi: 10.1016/j.msard.2021.103431. [DOI] [PubMed] [Google Scholar]
- 95.ClinicalTrials.gov. Short-term B-cell Depletion in Relapsing Multiple Sclerosis. https://clinicaltrials.gov/ct2/show/NCT03853746. Accessed 13 Sep 2022.
- 96.ClinicalTrials.gov. Effects of Ocrelizumab on B-cell Tolerance Defect in Relapsing Multiple Sclerosis. https://clinicaltrials.gov/ct2/show/NCT04261790. Accessed 13 Sep 2022.
- 97.Häusler D, Häusser-Kinzel S, Feldmann L, Torke S, Lepennetier G, Bernard CCA, et al. Functional characterization of reappearing B cells after anti-CD20 treatment of CNS autoimmune disease. Proc Natl Acad Sci U S A. 2018;115:9773–9778. doi: 10.1073/pnas.1810470115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Novartis Pharmaceuticals Corporation. GILENYA® (fingolimod) [package insert]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/022527s031lbl.pdf. Accessed 11 Aug 2022.
- 99.Hatcher SE, Waubant E, Nourbakhsh B, Crabtree-Hartman E, Graves JS. Rebound syndrome in patients with multiple sclerosis after cessation of fingolimod treatment. JAMA Neurol. 2016;73:790–794. doi: 10.1001/jamaneurol.2016.0826. [DOI] [PubMed] [Google Scholar]
- 100.Goncuoglu C, Tuncer A, Bayraktar-Ekincioglu A, Ayvacioglu Cagan C, Acar-Ozen P, Cakan M, et al. Factors associated with fingolimod rebound: a single center real-life experience. Mult Scler Relat Disord. 2021;56:103278. doi: 10.1016/j.msard.2021.103278. [DOI] [PubMed] [Google Scholar]
- 101.Prosperini L, Kinkel RP, Miravalle AA, Iaffaldano P, Fantaccini S. Post-natalizumab disease reactivation in multiple sclerosis: systematic review and meta-analysis. Ther Adv Neurol Disord. 2019;12:1756286419837809. doi: 10.1177/1756286419837809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Papeix C, Vukusic S, Casey R, Debard N, Stankoff B, Mrejen S, TYSEDMUS and OFSEP Group et al. Risk of relapse after natalizumab withdrawal: results from the French TYSEDMUS cohort. Neurol Neuroimmunol Neuroinflamm. 2016;3:e297. doi: 10.1212/NXI.0000000000000297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fuentes-Rumí L, Hernández-Clares R, Carreón-Guarnizo E, Valero-López G, Iniesta-Martinez F, Cabrera-Maqueda JM, et al. Prevention of rebound effect after natalizumab withdrawal in multiple sclerosis. Study of two high-dose methylprednisolone schedules. Mult Scler Relat Disord. 2020;44:102311. doi: 10.1016/j.msard.2020.102311. [DOI] [PubMed] [Google Scholar]
- 104.Rowles WM, Hsu W-Y, McPolin K, Li A, Merrill S, Guo CY, et al. Transitioning from S1P receptor modulators to B cell-depleting therapies in multiple sclerosis: clinical, radiographic, and laboratory data. Neurol Neuroimmunol Neuroinflamm. 2022;9:e1183. doi: 10.1212/NXI.0000000000001183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bloomgren G, Richman S, Hotermans C, Subramanyam M, Goelz S, Natarajan A, et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med. 2012;366:1870–1880. doi: 10.1056/NEJMoa1107829. [DOI] [PubMed] [Google Scholar]
- 106.Smith TE, Kister I. Infection mitigation strategies for multiple sclerosis patients on oral and monoclonal disease-modifying therapies. Curr Neurol Neurosci Rep. 2021;21:36. doi: 10.1007/s11910-021-01117-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ho P-R, Koendgen H, Campbell N, Haddock B, Richman S, Chang I. Risk of natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: a retrospective analysis of data from four clinical studies. Lancet Neurol. 2017;16:925–933. doi: 10.1016/S1474-4422(17)30282-X. [DOI] [PubMed] [Google Scholar]
- 108.Ryerson LZ, Foley J, Chang I, Kister I, Cutter G, Metzger RR, et al. Risk of natalizumab-associated PML in patients with MS is reduced with extended interval dosing. Neurology. 2019;93:e1452–e1462. doi: 10.1212/WNL.0000000000008243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ryerson LZ, Li X, Goldberg JD, Hoyt T, Christensen A, Metzger RR, et al. Pharmacodynamics of natalizumab extended interval dosing in MS. Neurol Neuroimmunol Neuroinflamm. 2020;7:e672. doi: 10.1212/NXI.0000000000000672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ryerson LZ, Foley J, Chang I, Kister I, Cutter G, Metzger R, et al. Natalizumab extended interval dosing (EID) is associated with a reduced risk of progressive multifocal leukoencephalopathy (PML) than every-4-week (Q4W) dosing: updated analysis of the TOUCH® Prescribing Program database (1988) Neurology. 2020;94:1988. [Google Scholar]
- 111.Ryerson LZ, Foley J, Kister I, Cutter G, Metzger R, Goldberg JD, et al. Natalizumab extended interval dosing (EID) is associated with a reduced risk of progressive multifocal leukoencephalopathy (PML) compared with every-4-week (Q4W) dosing: updated analysis of the TOUCH® Prescribing Program Database (4419) Neurology. 2021;96:4419. [Google Scholar]
- 112.Foley JF, Defer G, Ryerson LZ, Cohen JA, Arnold DL, Butzkueven H, NOVA study investigators et al. Comparison of switching to 6-week dosing of natalizumab versus continuing with 4-week dosing in patients with relapsing-remitting multiple sclerosis (NOVA): a randomised, controlled, open-label, phase 3b trial. Lancet Neurol. 2022;21:608–619. doi: 10.1016/S1474-4422(22)00143-0. [DOI] [PubMed] [Google Scholar]
- 113.Toorop AA, van Lierop ZYG, Strijbis EEM, Teunissen CE, Petzold A, Wattjes MP, et al. Mild progressive multifocal leukoencephalopathy after switching from natalizumab to ocrelizumab. Neurol Neuroimmunol Neuroinflamm. 2021;8:e904. doi: 10.1212/NXI.0000000000000904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Luna G, Alping P, Burman J, Fink K, Fogdell-Hahn A, Gunnarsson M, et al. Infection risks among patients with multiple sclerosis treated with fingolimod, natalizumab, rituximab, and injectable therapies. JAMA Neurol. 2020;77:184–191. doi: 10.1001/jamaneurol.2019.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Patel A, Sul J, Gordon ML, Steinklein J, Sanguinetti S, Pramanik B, et al. Progressive multifocal leukoencephalopathy in a patient with progressive multiple sclerosis treated with ocrelizumab monotherapy. JAMA Neurol. 2021;78:736–740. doi: 10.1001/jamaneurol.2021.0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gerevini S, Capra R, Bertoli D, Sottini A, Imberti L. Immune profiling of a patient with alemtuzumab-associated progressive multifocal leukoencephalopathy. Mult Scler. 2019;25:1196–1201. doi: 10.1177/1352458519832259. [DOI] [PubMed] [Google Scholar]
- 117.National Multiple Sclerosis Society. Multiple sclerosis & COVID-19. https://www.nationalmssociety.org/coronavirus-covid-19-information/multiple-sclerosis-and-coronavirus#section-0. Accessed 1 Feb 2022.
- 118.Multiple Sclerosis International Federation. MS, the coronavirus and vaccines – updated global advice. https://www.msif.org/news/2020/02/10/the-coronavirus-and-ms-what-you-need-to-know/. Accessed 1 Feb 2022.
- 119.Reder AT, Centonze D, Naylor ML, Nagpal A, Rajbhandari R, Altincatal A, et al. COVID-19 in patients with multiple sclerosis: associations with disease-modifying therapies. CNS Drugs. 2021;35:317–330. doi: 10.1007/s40263-021-00804-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Simpson-Yap S, De Brouwer E, Kalincik T, Rijke N, Hillert JA, Walton C, et al. Associations of disease-modifying therapies with COVID-19 severity in multiple sclerosis. Neurology. 2021;97:e1870–e1885. doi: 10.1212/WNL.0000000000012753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cross A, Delgado S, Habek M, Davydovskaya M, Ward BJ, Cree BAC, et al. Outcomes of COVID-19 in patients with relapsing multiple sclerosis receiving ofatumumab: data from the ALITHIOS study and post-marketing surveillance. Presented at: 37th Congress of the European Committee for Treatment and Research in Multiple Sclerosis; 13–15 October 2021; Virtual.
- 122.Jack D, Damian D, Nolting A, Galazka A. COVID-19 in patients with multiple sclerosis treated with cladribine tablets: an update. Mult Scler Relat Disord. 2021;51:102929. doi: 10.1016/j.msard.2021.102929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Giovannoni G, Boster AL, Berkovich R, Selmaj KW, Chung L, Shiota D, et al. Cases of COVID-19 in alemtuzumab-treated patients with MS: pharmacovigilance report (2059) Neurology. 2021;96:4. [Google Scholar]
- 124.Sabatino JJ, Mittl K, Rowles W, McPolin K, Rajan JV, Zamecnik CR, et al. Impact of multiple sclerosis disease-modifying therapies on SARS-CoV-2 vaccine-induced antibody and T cell immunity. medRxiv. 2021 doi: 10.1101/2021.09.10.21262933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Brill L, Rechtman A, Zveik O, Haham N, Oiknine-Djian E, Wolf DG, et al. Humoral and T-cell response to SARS-CoV-2 vaccination in patients with multiple sclerosis treated with ocrelizumab. JAMA Neurol. 2021;78:1510–1514. doi: 10.1001/jamaneurol.2021.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Apostolidis SA, Kakara M, Painter MM, Goel RR, Mathew D, Lenzi K, et al. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat Med. 2021;27:1990–2001. doi: 10.1038/s41591-021-01507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Disanto G, Sacco R, Bernasconi E, Martinetti G, Keller F, Gobbi C, et al. Association of disease-modifying treatment and anti-CD20 infusion timing with humoral response to 2 SARS-CoV-2 vaccines in patients with multiple sclerosis. JAMA Neurol. 2021;78:1529–1531. doi: 10.1001/jamaneurol.2021.3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sormani MP, Inglese M, Schiavetti I, Carmisciano L, Laroni A, Lapucci C, CovaXiMS study group on behalf of the Italian Covid-19 Alliance in MS et al. Effect of SARS-CoV-2 mRNA vaccination in MS patients treated with disease modifying therapies. EBioMedicine. 2021;72:103581. doi: 10.1016/j.ebiom.2021.103581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Oreja-Guevara C, Ramirez CI, Alba-Suarez E, Gomez-Estevez I, Bullón-Sanchez C, Castro-Hernandez M, et al. COVID-19 vaccination and humoral immune response in people with multiple sclerosis. Mult Scler. 2021;27:728. [Google Scholar]
- 130.National Multiple Sclerosis Society. COVID-19 vaccine guidance for people living with MS. https://www.nationalmssociety.org/coronavirus-covid-19-information/multiple-sclerosis-and-coronavirus/covid-19-vaccine-guidance. Accessed 1 Feb 2022.
- 131.National Multiple Sclerosis Society. Timing MS medications with COVID-19 mRNA vaccines. https://www.nationalmssociety.org/coronavirus-covid-19-information/multiple-sclerosis-and-coronavirus/covid-19-vaccine-guidance/Timing-MS-Medications-with-COVID-19-mRNA-Vaccines. Accessed 1 Feb 2022.
- 132.Melamed E, Lee MW. Multiple sclerosis and cancer: the ying-yang effect of disease modifying therapies. Front Immunol. 2020;10:2954. doi: 10.3389/fimmu.2019.02954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pakpoor J, Disanto G, Altmann DR, Pavitt S, Turner BP, Marta M, et al. No evidence for higher risk of cancer in patients with multiple sclerosis taking cladribine. Neurol Neuroimmunol Neuroinflamm. 2015;2:e158. doi: 10.1212/NXI.0000000000000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Leist T, Cook S, Comi G, Montalban X, Giovannoni G, Nolting A, et al. Long-term safety data from the cladribine tablets clinical development program in multiple sclerosis. Mult Scler Relat Disord. 2020;46:102572. doi: 10.1016/j.msard.2020.102572. [DOI] [PubMed] [Google Scholar]
- 135.Coles AJ, Cohen JA, Fox EJ, Giovannoni G, Hartung HP, Havrdova E, CARE-MS II and CAMMS03409 Investigators et al. Alemtuzumab CARE-MS II 5-year follow-up: efficacy and safety findings. Neurology. 2017;89:1117–1126. doi: 10.1212/WNL.0000000000004354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Havrdova E, Arnold DL, Cohen JA, Hartung H-P, Fox EJ, Giovannoni G, CARE-MS I and CAMMS03409 Investigators et al. Alemtuzumab CARE-MS I 5-year follow-up: durable efficacy in the absence of continuous MS therapy. Neurology. 2017;89:1107–1116. doi: 10.1212/WNL.0000000000004313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fleury I, Chevret S, Pfreundschuh M, Salles G, Coiffier B, van Oers MH, et al. Rituximab and risk of second primary malignancies in patients with non-Hodgkin lymphoma: a systematic review and meta-analysis. Ann Oncol. 2016;27:390–397. doi: 10.1093/annonc/mdv616. [DOI] [PubMed] [Google Scholar]
- 138.Butzkueven H, Kappos L, Wiendl H, Trojano M, Spelman T, Chang I, Tysabri Observational Program (TOP) Investigators et al. Long-term safety and effectiveness of natalizumab treatment in clinical practice: 10 years of real-world data from the Tysabri Observational Program (TOP) J Neurol Neurosurg Psychiatry. 2020;91:660–668. doi: 10.1136/jnnp-2019-322326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Coles AJ, Jones JL, Vermersch P, Traboulsee A, Bass AD, Boster A, et al. Autoimmunity and long-term safety and efficacy of alemtuzumab for multiple sclerosis: benefit/risk following review of trial and post-marketing data. Mult Scler. 2022;28:842–846. doi: 10.1177/13524585211061335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.US Food and Drug Administration. FDA warns about rare but serious risks of stroke and blood vessel wall tears with multiple sclerosis drug Lemtrada (alemtuzumab). https://www.fda.gov/drugs/fda-drug-safety-podcasts/fda-warns-about-rare-serious-risks-stroke-and-blood-vessel-wall-tears-multiple-sclerosis-drug#:~:text=On%20November%2029%2C%202018%20FDA,permanent%20disability%20and%20even%20death. Accessed 21 Jan 2022.
- 141.Giovannoni G, Galazka A, Schick R, Leist T, Comi G, Montalban X, et al. Pregnancy outcomes during the clinical development program of cladribine in multiple sclerosis: an integrated analysis of safety. Drug Saf. 2020;43:635–643. doi: 10.1007/s40264-020-00948-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Carnero Contentti E, Correale J. Bruton’s tyrosine kinase inhibitors: a promising emerging treatment option for multiple sclerosis. Exp Opin Emerg Drugs. 2020;25:377–381. doi: 10.1080/14728214.2020.1822817. [DOI] [PubMed] [Google Scholar]
- 143.Pender MP, Csurhes PA, Smith C, Douglas NL, Neller MA, Matthews KK, et al. Epstein-Barr virus-specific T cell therapy for progressive multiple sclerosis [abstract] JCI Insight. 2018;3:e124714. doi: 10.1172/jci.insight.124714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pender M, Hodgkinson S, Broadley S, Lindsey J, Ioannides Z, Munson D, et al. Preliminary safety and efficacy of ATA188, a pre-manufactured, unrelated donor (off-the-shelf, allogeneic) Epstein-Barr virus-targeted T-cell immunotherapy for patients with progressive forms of multiple sclerosis. Mult Scler. 2019;25:P1657. [Google Scholar]
- 145.Atkins HL, Bowman M, Allan D, Anstee G, Arnold DL, Bar-Or A, et al. Immunoablation and autologous haemopoietic stem-cell transplantation for aggressive multiple sclerosis: a multicentre single-group phase 2 trial. Lancet. 2016;388:576–585. doi: 10.1016/S0140-6736(16)30169-6. [DOI] [PubMed] [Google Scholar]
- 146.Burt RK, Balabanov R, Burman J, Sharrack B, Snowden JA, Oliveira MC, et al. Effect of nonmyeloablative hematopoietic stem cell transplantation vs continued disease-modifying therapy on disease progression in patients with relapsing-remitting multiple sclerosis: a randomized clinical trial. JAMA. 2019;321:165–174. doi: 10.1001/jama.2018.18743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ge F, Lin H, Li Z, Chang T. Efficacy and safety of autologous hematopoietic stem-cell transplantation in multiple sclerosis: a systematic review and meta-analysis. Neurol Sci. 2019;40:479–487. doi: 10.1007/s10072-018-3670-1. [DOI] [PubMed] [Google Scholar]
- 148.Sormani MP, Muraro PA, Schiavetti I, Signori A, Laroni A, Saccardi R, et al. Autologous hematopoietic stem cell transplantation in multiple sclerosis: a meta-analysis. Neurology. 2017;88:2115–2122. doi: 10.1212/WNL.0000000000003987. [DOI] [PubMed] [Google Scholar]
- 149.Cohen JA, Baldassari LE, Atkins HL, Bowen JD, Bredeson C, Carpenter PA, et al. Autologous hematopoietic cell transplantation for treatment-refractory relapsing multiple sclerosis: position statement from the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2019;25:845–854. doi: 10.1016/j.bbmt.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 150.Miller AE, Chitnis T, Cohen BA, Costello K, Sicotte NL, Stacom R, National Medical Advisory Committee of the National Multiple Sclerosis Society Autologous hematopoietic stem cell transplant in multiple sclerosis: recommendations of the National Multiple Sclerosis Society. JAMA Neurol. 2021;78:241–246. doi: 10.1001/jamaneurol.2020.4025. [DOI] [PubMed] [Google Scholar]
- 151.Sharrack B, Saccardi R, Alexander T, Badoglio M, Burman J, Farge D, European Society for Blood and Marrow Transplantation (EBMT) Autoimmune Diseases Working Party (ADWP) and the Joint Accreditation Committee of the International Society for Cellular Therapy ISCT and EBMT (JACIE) et al. Autologous haematopoietic stem cell transplantation and other cellular therapy in multiple sclerosis and immune-mediated neurological diseases: updated guidelines and recommendations from the EBMT Autoimmune Diseases Working Party (ADWP) and the Joint Accreditation Committee of EBMT and ISCT (JACIE) Bone Marrow Transplant. 2020;55:283–306. doi: 10.1038/s41409-019-0684-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chung KK, Altmann D, Barkhof F, Miszkiel K, Brex PA, O'Riordan J, et al. A 30-year clinical and magnetic resonance imaging observational study of multiple sclerosis and clinically isolated syndromes. Ann Neurol. 2020;87:63–74. doi: 10.1002/ana.25637. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.