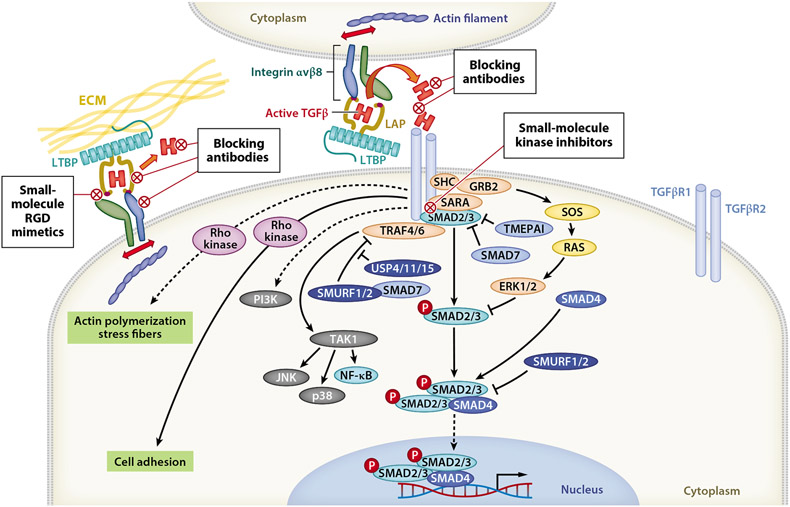
Figure 1.
TGFβ signaling pathway showing druggable targets. The TGFβ receptors are dual-specificity kinases capable of phosphorylating serine/threonine and tyrosine residues. The canonical SMAD signaling pathway requires ligand-induced kinase activity of TGFβR2, which phosphorylates TGFβR1. TGFβR1 then phosphorylates the receptor-associated SMADs, SMAD2 and SMAD3. Phosphorylated SMAD2/3 forms a hexameric complex with SMAD4 and shuttles to the nucleus to initiate transcriptional responses that are context dependent and influenced by the availability of other transcription factors and cofactors. The TGFβ receptors can also directly activate other non-SMAD signaling pathways, including PI3K/AKT/mTORC, JNK, p38 MAPK, MEK/ERK, NF-κB/JAK/STAT, and Rho kinases. These pathways are activated by TGFβ binding to its receptors within distinct subcellular compartments (caveolae versus clathrin-coated pits), often with slower kinetics, and with lower magnitude of signal transduction than their activation by other stimuli. Non-SMAD and SMAD signaling pathways compete; for example, several non-SMAD pathways require SHCA binding to TGFβR1, and SHCA competes with R-SMADs for binding to TGFβR1. SARA potentiates R-SMAD binding to TGFβR1, while SMAD7 and TMEPAI antagonize this binding. Whereas the SHCA/GRB2/RAS/ERK pathway depends on TGFβR1 kinase activity, TRAF4/6 mediates ligand-activated signaling of JNK, p38 MAPK, and NF-κB pathways independent of TGFβR1 kinase activity. In this case, TGFβ induces recruitment of TAK1 to the type I receptor by its association with TRAF4 or TRAF6, which are RING domain E3 ubiquitin kinases. TRAF4/6 is activated by ligand-induced conformational changes in TGFβR1, causing ubiquitination and consequent activation of this kinase and its downstream pathways. Ubiquitination of TRAF4/6 and of SMADs by SMURF1/2 results in degradation of these targets, with USP deubiquitinases counteracting this activity. Abbreviations: ECM, extracellular matrix; LAP, latency-associated peptide; LTBP, latent TGFβ-binding protein; RGD, arginylglycylaspartic acid; TGFβR1, TGFβ receptor type 1; TGFβR2, TGFβ receptor type 2.