Abstract
Background:
There are some adverse effects with coronavirus disease 2019 (COVID-19) vaccines, but the impact of COVID-19 vaccination on attacks in hereditary angioedema (HAE) is not well defined.
Objective:
We aimed to investigate the influence of COVID-19 vaccination on the course of HAE.
Method:
The COVID-19 vaccination status was determined in 140 adult patients with HAE. The number and severity of attacks recorded from patients' diaries were evaluated at four different periods, comprising 1 month before the first dose, the period between the first and the second doses of COVID-19 vaccine in all the patients, the period between the second dose and the third doses in those who received three doses, and 1 month after the last vaccination dose. The disease and attack severities were assessed with the disease severity score (DSS) and 10-point visual analog scale, respectively. The patients were divided into two main groups as group 1 (those who had at least two doses of COVID-19 vaccines [n = 114]) and group 2 (those who had no vaccination [n = 26]). Only Sinovac and Biontech, which were only approved in Turkey.
Results:
The mean ± standard deviation DSS was significantly higher in the patients who experienced an attack after vaccination within 48 hours (6.61 ± 1.88 versus 4.14 ± 1.69; p < 0.001). Long-term prophylaxis was less common in the patients with an increased number of attacks (n = 5 (27.8%) versus n = 54 (56.3%); p = 0.027). The number of patients with less than a high school education was higher in group 2 (n = 23 [88.5%]) than in group 1 (n = 26 [3.1%]) (p < 0.001). The number of patients who had concerns about the triggering of a vaccine-induced HAE attack or about the possible vaccine adverse effects was higher in group 2 (n = 26 [100%]) than in group 1 (n = 74 [64.9%]).
Conclusion:
It seems that COVID-19 vaccination does not increase HAE attacks regardless of the type of the vaccines. We recommend that HAE activity should be under control before COVID-19 vaccination, and the patients should be well informed about the safety of the vaccines.
Keywords: Hereditary angioedema, COVID-19, Biontech, Sinovac, attacks, long term prophylaxis, disease severity, education, side effect, vaccine impact
Hereditary angioedema (HAE) is a rare genetic disorder characterized by subcutaneous, submucosal, or mucosal swelling attacks without pruritus.1 HAE is a disease with deficient C1 inhibitor (C1-INH) (type 1) or with dysfunctional C1-INH (type 2) or disease-associated mutations with normal C1-INH level and/or function HAE.1 Attacks can be life-threatening and can spontaneously occur.2,3 Furthermore, infections; weather changes; physical exertion; emotional stress; anxiety; hormonal changes; and some medications, such as oral contraceptive pills that contain estrogen or angiotensin-converting enzyme inhibitors, could be triggers of the attacks in HAE.2,3–5 The role of any vaccination on the course of HAE has not been studied before.
Since the beginning of the coronavirus disease 19 (COVID-19) pandemic, vaccination has gained importance worldwide.6 As of April 2022, four COVID-19 vaccines were approved by the Turkish Ministry of Health in Turkey.7 The first approved COVID-19 vaccine in Turkey is an inactive virus vaccine, Coronovac (Sinovac, Pekin, China), first given to health-care workers and patients who had a chronic systemic disease or to those ages > 65 years.7 The second approved COVID-19 vaccine is a modified messenger RNA vaccine, Commirnaty (Pfizer/Biontech, Mainz, Germany),7 which is given to all the population as a first and subsequent two doses, or to people who had two Sinovac vaccines as a reminder.7 Although a vector vaccine, Sputnik (Gamaleya National Institute of Epidemiology and Microbiology, Russia), and an inactive virus vaccine, Turkovac (Dollvet, Sanlıurfa, Turkey), were approved in Turkey, Sputnik was not used, and Turkovac was recently introduced.7
To date, there are some well-known adverse effects of Sinovac and Pfizer/Biontech vaccines, such as injection-site reactions, fever, fatigue, or headache8,9 but the impact of COVID-19 vaccination itself or its adverse effects on the HAE attacks is not well defined.10 Although vaccination is crucial to control the pandemic, people with HAE may have some concerns about vaccination due to a fear of adverse effects and the possibility of leading to angioedema attacks and deterioration in the disease course.11 In this study, we aimed to investigate the influence of COVID-19 vaccination on attack frequency and the course of HAE.
METHOD
Patient Selection and Study Design
This retrospective study included 140 patients with HAE who were >18 years old and were followed up at the outpatient allergy clinic, the tertiary reference center for HAE, and an ACARE center in the Istanbul Faculty of Medicine. HAE was diagnosed and classified according to the International World Allergy Organization (WAO)/European Academy of Allergy and Clinical Immunology (EAACI) guideline for HAE management.1 Vaccination status was determined from patients' vaccination cards. Patients who had at least two doses of COVID-19 vaccines were included for the analysis of the vaccinated group. The patients were divided into two main groups according to being vaccinated or not vaccinated as group 1 (those who were vaccinated with at least two doses of COVID-19 vaccine [n = 114 {81.4%}]) and group 2 (those who did not receive vaccination [n = 26 {18.6%}]). Group 1 was further allocated as group 1a (those who had two doses of COVID-19 vaccines [n = 48 {42.1%}]) and group 1b (those who had three doses of COVID-19 vaccines [n = 66 {57.9%}]).
The patients who were <18 years old and who did not informed consent were excluded from the study. Also, the patients who had normal C1-INH HAE were excluded from the study due to having a different mechanism for HAE attacks. For evaluating the possible major adverse effect of vaccination on the course of HAE attacks, the study was composed of four periods: 1 month before the first dose (P1), the period between the first and second dose in all the patients (P2), the period between the second and third dose in the patients who had three doses of COVID-19 vaccination (P3), and 1 month after all vaccination in the patients who received at least two doses of vaccination (P4).
Clinical Data Collection
Patient assessments 1 month before the first dose of vaccination included the knowledge of the number and severity of HAE attacks, the history of a COVID-19 infection, COVID-19 infection related frequency, and severity of HAE attacks, which were questioned by the physicians according to the data in patients' diaries. Also, the demographic and clinical features of the patients were collected from the patients' medical charts. When education status was compared, a higher education level was defined as being a high school or university graduate.
Collection of Vaccination Data
After each dose of COVID-19 vaccines in the patients who were vaccinated, vaccine types and doses, and having any concern about vaccination were retrospectively collected from patients' diaries and medical charts.
Collection of Vaccine-Related Adverse Effects Data
The adverse effects of vaccination were evaluated from the medical charts or emergency records retrospectively.
Evaluating Attacks after Vaccination
HAE attacks, including the time interval between the attack after the vaccination, attack frequency, severity, and related treatment were collected from patients' diaries and medical charts. Attack severity was evaluated with 10-point visual analog scale (VAS), and disease severity was assessed with the general disease severity score (DSS).12,13 DSS is a general disease score that was developed by Bygum et al.12 and was used successfully to assess the disease severity for HAE. A DSS < 7 was considered to be mild to moderate and ≥7 was considered as a severe disease.12 VAS score in the worst attack was defined as the highest VAS score in that attack. Treatment options and long-term prophylaxis (LTP) were collected from the patients' medical charts and diaries. İstanbul University, İstanbul Faculty of Medicine Ethics Committee approved this study (approval 834617) in accordance with the Declaration of Helsinki, and written informed consent was received from all participants. The author contributions included the following: N. Öztop, S. Demir, İ.D. Toprak, D. Ünal, and A. Gelincik have made substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data; have been involved in drafting the manuscript or revising it critically for important intellectual content.
Statistical Analysis
The data were analyzed by using the Statistical Package for Social Sciences v25.0 (SPSS Inc. Armonk, NY), and GraphPad Prism Software 8 (San Diego, CA) was used for obtaining the figures. According to the data distribution, demographic and clinical features were assessed by descriptive analysis and are shown as percentages and mean ± standard deviation (SD) or as median (interquartile range, 25–75 percentiles [IQR]). The Kolmogorov–Smirnov test was conducted to assess the distribution pattern of the quantitative data. Continuous variables were compared by conducting an independent t-test or a Mann-Whitney U test between the groups. The Wilcoxon rank test and the paired sample t-test were used to compare the dependent means. The p values < 0.05 were considered statistically significant.
RESULTS
Results of Demographics and Clinical Characteristics of the Patients
The mean ± SD age of the patients was 38.51 ± 12.92 years, and 91 (65%) were women. Although 136 of the patients (97.1%) had type I HAE, 4 (2.9%) had type II HAE; 91 of the patients (65%) had a higher education level; 30 (21.4%) had concomitant diseases, including diabetes (n = 9 [6.4%]), hypertension (n = 9 [6.4%]), cardiac diseases (n = 2 [1.4%]), thyroid disease (n = 6 [4.3%]), malignancy history (n = 1 [0.7%]), or other diseases (n = 10 [7.1%]); 100 of the patients (71.4%) had concerns about vaccination, including possible deterioration effects of vaccination on the course of HAE or adverse effects. A total of 45 patients (32.1%) had a COVID-19 history in all the patients included in the study, and there was no COVID-19 history after vaccination among group 1. The patient demographics and clinical characteristics are summarized in Table 1.
Table 1.
Demographics and clinical characteristics of the patients (N = 140)
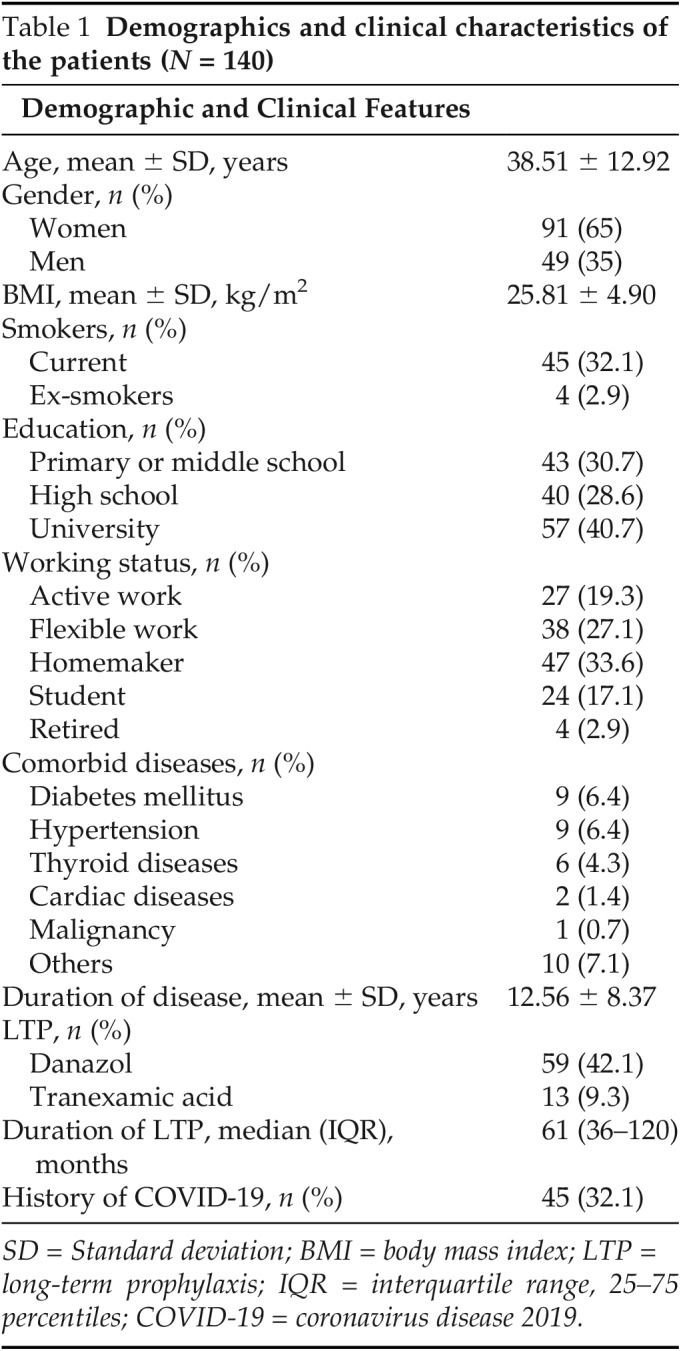
SD = Standard deviation; BMI = body mass index; LTP = long-term prophylaxis; IQR = interquartile range, 25–75 percentiles; COVID-19 = coronavirus disease 2019.
Comparison Analysis between Patients with or Those without Vaccination
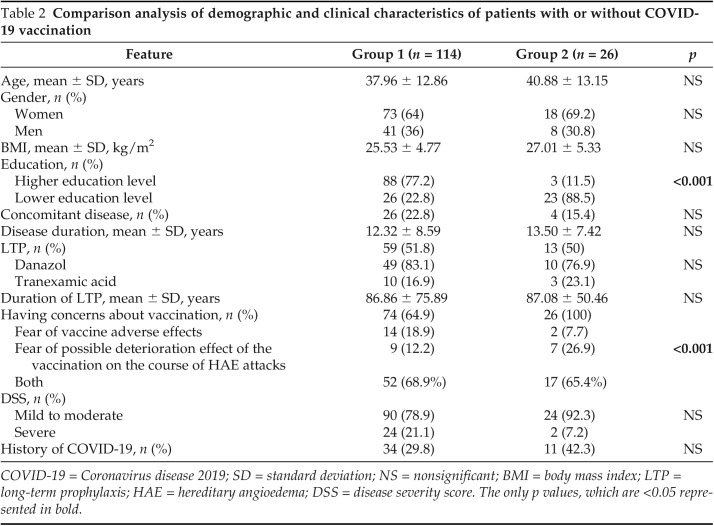
Sixty patients (42.9%) received Pfizer/Biontech vaccine alone, and 28 (20%) received Sinovac vaccine alone, 26 (18.6%) had both Pfizer/Biontech and Sinovac vaccines in group 1; in addition, none of the patients received only one dose of COVID vaccine in our study. The patients in group 1 had a higher education level versus those in group 2 (n = 88 [77.2%] versus n = 3 [11.5%]; p < 0.001]). Furthermore, there was a significant correlation between a higher education level and vaccination rate (r = 0.494; p < 0.001). Concerns with regard to the possible deterioration effect of vaccination on the course of HAE or adverse effects were higher in group 2 versus in group 1 (n = 74 [64.9%] versus n = 26 [100%]; p < 0.001]. There were no differences with regard to age, gender, and DSS, and to having a concomitant disease history between the two groups. Comparison analysis of the demographic and clinical characteristics of group 1 and group 2 are summarized in Table 2.
Table 2.
Comparison analysis of demographic and clinical characteristics of patients with or without COVID-19 vaccination
COVID-19 = Coronavirus disease 2019; SD = standard deviation; NS = nonsignificant; BMI = body mass index; LTP = long-term prophylaxis; HAE = hereditary angioedema; DSS = disease severity score. The only p values, which are <0.05 represented in bold.
Subgroup Analysis of Patients Who Were Vaccinated
The patients were questioned about the decision of vaccination, and 80 patients (70.2%) decided to receive the vaccine by their own opinion, 29 patients (25.4%) were vaccinated with a physician's advice, and 5 patients (4.4%) were vaccinated in accordance with the legal rules at the workplace. Thirty-three patients (68.8%) received a Pfizer/Biontech vaccination alone and 15 patients (31.2%) received a Sinovac vaccination alone in group 1a; 27 patients (40.9%), 13 (19.7%), and 26 (39.4%) received a Pfizer/Biontech vaccination alone, Sinovac vaccination alone, and Biontech or Sinovac vaccination in different doses in group 1b, respectively.
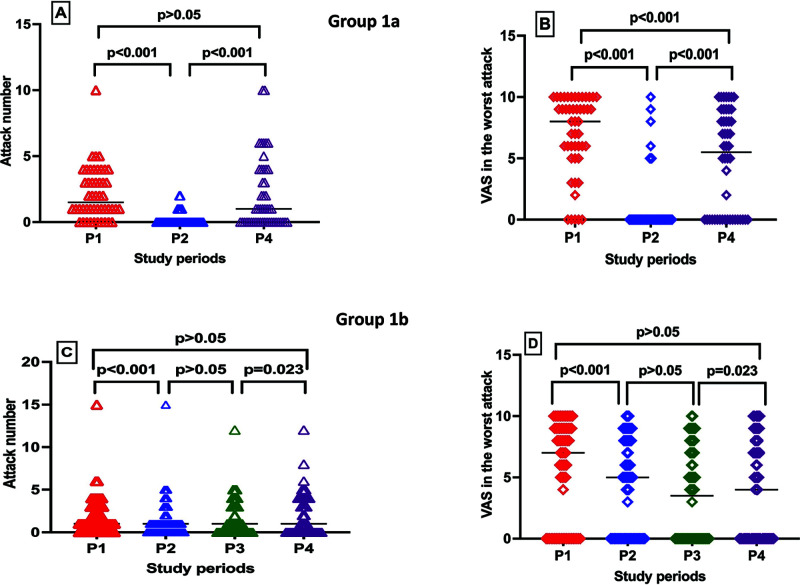
There were no significant differences in gender, education level, concomitant diseases history, having concern about vaccination, DSS, adverse effects after vaccination, or increased attacks after vaccination between group 1a and group 1b. In the comparison analysis of the number and severity of HAE attacks in each period, the attack numbers (median [IQR]) were fewer in P2 (0 [0-0]) than in P1 (1.5 [1–3.75]) and P4 (1 [0–4]) (p < 0.001), whereas no significant difference was seen between P1 and P4 (p = 0.839) (Fig. 1 A), and the median (IQR) VAS scores in the worst attack was 8 (5.25–10), 0 (0–0), and 5.5 (0–8.75) in P1, P2, and P4, respectively (p < 0.001 for P1-P2, P2-P4, P1-P4), in group 1a (Fig. 1 B).
Figure 1.
A comparison of HAE attack numbers (A, C) and VAS scores in the worst attack (B, D) in each period between group 1a and group 1b. HAE = Hereditary angioedema; VAS = visual analog scale; P1 = period 1, 1 month before the first dose; P2, period 2, the period between the first and the second doses of coronavirus disease 2019 (COVID-19) vaccine in all the patients; P3, period 3, the period between the second dose and the third dose in those who received three doses; P4, period 4, 1 month after the last vaccination dose.
In addition, the comparison analysis of HAE attack numbers in each period in group 1b and the median (IQR) attack numbers were 1 (0–3), 1 (0–1), 1 (0–3), and 1 (0–4) in P1, P2, P3, and P4, respectively, and the differences were significant among both P1 and P2 and P3 and P4 (p < 0.001; p = 0.023, respectively), but there was no difference between attack numbers between P2 and P3 and P1 and P4 (Fig. 1 C). The median (IQR) VAS scores in the worst attack were 7 (0–9), 5 (0–8), 3.5 (0–7), and 4 (0–8) in P1, P2, P3, and P4, respectively (p < 0.001, p = 0.04, p = 0.154, p < 0.001 for P1-P2, P2-P3, P3-P4, and P1-P4, respectively) in group 1b (Fig. 1 D). The attack numbers and VAS scores in the worst attack were compared between group 1a and group1b in each period. There was no significant difference for the attack numbers and VAS scores in the worst attack between the two groups before the first dose and after the last dose of vaccination periods; however, the median attack numbers and VAS scores in the worst attack were higher in group 1b than in group 1a in P2 (<0.001).
Vaccine Adverse Effects
A total of 48 patients (42.1%) had vaccine-related adverse effects. The most common adverse effect was injection-site reactions (n = 32 [66.7%]), such as pain, erythema, or swelling. Headache, myalgia, and fever were seen in 2 patients (4.2%), 5 (10.4%), and 9 (18.7%), respectively. Although 11 patients (22.9%) had adverse effects after the first dose of vaccine, 6 patients (12.5%) had adverse effects after the second dose, 2 patients (4.2%) had adverse effects after the third dose, and 29 patients had adverse effects both after the first and second doses of vaccine. In addition, 39 patients (81.3%) had vaccine-related adverse effects within 6 hours and 9 patients (18.2%) had vaccine-related adverse effects after 12 hours. Among the patients who developed vaccine-related adverse effects, 44 patients had at least one dose of the Pfizer/Biontech vaccine, and 13 patients had at least one dose of the Sinovac vaccine. There were no vaccine-related severe adverse effects.
Analysis Impact of Vaccination on the Course of HAE Attacks
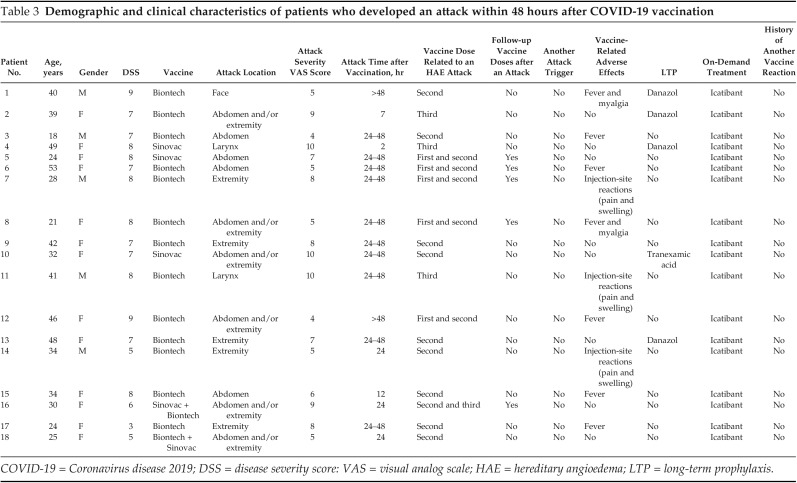
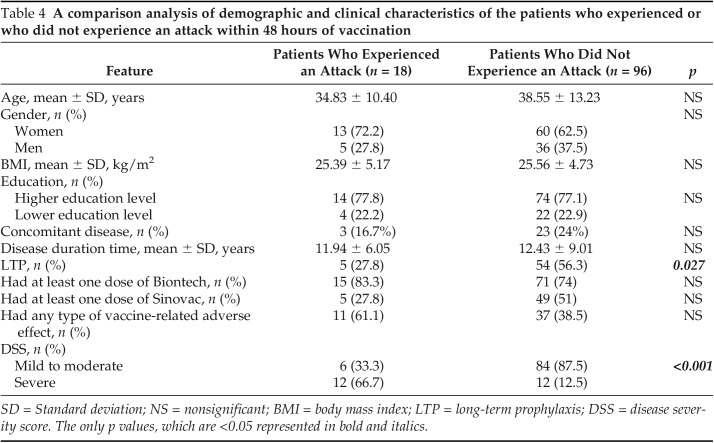
Eighteen patients (15.8%) experienced an HAE attack within 48 hours after COVID-19 vaccination. Demographics and clinical characteristics of these patients are shown in Table 3. The number of the patients who experienced attacks within 48 hours after vaccination was not different in group 1a versus group 1b (n = 10 [20.8%] versus n = 9 [13.6%]). The mean ± SD DSS was significantly higher in the patients who experienced attacks within 48 hours (6.61 ± 1.88 versus 4.14 ± 1.69; p < 0.001). Furthermore, the numbers of patients who received LTP were fewer among patients who had attacks after vaccination n = 5 (27.8%) versus n = 54 (56.3%); p = 0.027). There was no correlation between having any kind of vaccine-related adverse effect and HAE attacks after 48 hours after vaccination (p = 0.075). A comparison of the patients who had an attack with those without an attack are summarized in Table 4.
Table 3.
Demographic and clinical characteristics of patients who developed an attack within 48 hours after COVID-19 vaccination
COVID-19 = Coronavirus disease 2019; DSS = disease severity score: VAS = visual analog scale; HAE = hereditary angioedema; LTP = long-term prophylaxis.
Table 4.
A comparison analysis of demographic and clinical characteristics of the patients who experienced or who did not experience an attack within 48 hours of vaccination
SD = Standard deviation; NS = nonsignificant; BMI = body mass index; LTP = long-term prophylaxis; DSS = disease severity score. The only p values, which are <0.05 represented in bold and italics.
DISCUSSION
This study demonstrated that the vaccination rate is higher among the patients who were well educated, although they could have some concerns about the vaccination adverse effects and the impact of the vaccine on the course of HAE attacks. The number and severity of HAE attacks do not increase after COVID-19 vaccination and can even decrease between the first and second doses, as in our study. In patients who experience an attack after vaccination, disease severity documented by DSS can be higher, which indicated that patients who are severely ill can have a higher risk of attacks. However, we also observed that attacks after vaccination can be fewer among patients whose disease severity was lower. Therefore, we could infer that, before vaccination, the disease should be taken under control in patients whose disease severity is high.
Although the COVID-19 pandemic has affected the whole world in terms of health and economics, vaccines have been developed to provide pandemic control.14 During the pandemic, which could be reason of global health crisis, both patients and health-care professionals could have concerns that HAE attack control and treatment may be negatively affected.15 Developing a vaccine alone is not enough; people should accept being vaccinated. Therefore, the aim is to increase the vaccination rate in societies.14 Vaccine hesitancy is growing globally and may differ, depending on the type and origin of the vaccine.14,16 Allergists and immunologists especially should play an important role in raising public awareness of the importance of COVID-19 vaccines, in preventing misinformation, and in increasing confidence in vaccine acceptance.17
To date, May 2022, according to the Turkish Ministry of Health registry, the percentage of people who had at least one dose of any type of COVID-19 vaccine was 93.15%, and the percentage of people who had at least two doses of any type of COVID-19 vaccine was 85.44% in Turkey.18 In our study, we found that the vaccination rate among the patients with HAE was in line with the general population results. It was reported that a lower education level is related to fewer COVID-19 vaccinations.16,19–21 Similar to the literature, in our study, we found that the vaccination rate was lower among patients with HAE and who were less educated. In line with these findings, we may speculate that providing well-informed education on vaccine effectiveness and safety, covering the whole population, could increase the rate of vaccination to control the COVID-19 pandemic. Also, the current study is valuable because it indicates that vaccination against severe acute respiratory syndrome coronavirus 2 does not deteriorate the HAE disease course and so could be used to encourage the patients to get vaccinated.
Well-established triggers of HAE attacks include stress; infections; psychological or physical trauma, which comprise medical procedures such as dental or surgical operation; and drugs such as angiotensin-converting enzyme inhibitors or estrogen.1,22 The studies conducted on HAE attacks in the COVID-19 pandemic indicated that there was no increase in the severity of attacks directly related to the infection; however, an increase in attacks with stress created by the fear of COVID-19 infection was demonstrated.23,24 There is limited knowledge about the impact of COVID-19 vaccines on the course of HAE attacks. Fijen et al.10 reported that the COVID-19 vaccines, including messenger RNA and vector vaccines, can be used safely in HAE without short-term prophylaxis.
Similarly, in the current study, we found that COVID-19 vaccination did not increase HAE attacks, regardless of the type of the vaccines. In addition, we found that the mild or moderate attacks after vaccination were seen in patients with poorly controlled HAE. Also, in our study in a larger group of patients with HAE, we determined that LTP can reduce the rate of HAE attacks. With these findings, we could recommend that COVID-19 vaccines can be safely used without concerns of the occurrence of attacks in patients with HAE. Also, we can speculate that, to prevent HAE attacks, good disease control should be ensured before the vaccination and LTP should be started in patients with a history of frequent attacks.
The most common adverse effect of the two vaccines, Pfizer/Biontech and Sinovac, are reported to be local injection-site reactions, such as pain, erythema, or swelling followed by fever, myalgia, headache, and fatigue.8,9,25–27 In addition, hypersensitivity reactions have also been reported with COVID-19 vaccines, mostly with Pfizer/Biontech.26,28–30 In our study, similar to the literature, we determined that local reactions were the most common. Fortunately, our patients did not have any severe vaccine-related adverse effects, such as hypersensitivity reactions or death. In line with these findings, we could consider that the COVID-19 vaccines, including Sinovac and Pfizer/Biontech vaccines, are safe in patients with HAE in terms of adverse effects.
As a limitation of our study, due to not having published a Turkish version of Angioedema Control Test yet, we could have evaluated the disease severity with a generic tool DSS because a validated Turkish version of Angioedema Control Test has not been published yet. However, DSS was successfully used in previous HAE studies to assess the disease activity.12,31,32 When considering the situation, we believe that this tool was the most convenient to evaluate HAE activity. Another limitation of our study was that it was not possible to determine the long-term effects of COVID-19 vaccines on HAE attacks with our study periods. However, because infections, medications, or physical or psychological stress can easily trigger the attacks in these patients, it would not be easy to associate the attacks developed in the long term after vaccination as well as the vaccination numbers and periods between vaccination were not similar among patients.
Therefore, to make a cause-effect relationship between injections and attacks, and to standardize the periods for the evaluation of the impact of vaccines as much as possible in all the patients, we evaluated the relatively brief periods, including 1 month before and after vaccinations. As a last limitation, in the current study, we indicated that the disease control of the patients with increased attacks after vaccination was poor and that most of them were not under LTP. Although the reason of this finding could be the natural course of the disease, we could not ignore the association of vaccination because the majority of the attacks developed in 48 hours after injections. It would be better to compare the number of attacks before and after vaccination in patients with uncontrolled HAE and in those who are not receiving LTP; however, we did not have such a data.
CONCLUSION
COVID-19 vaccines, including Pfizer/Biontech and Sinovac, can be used in patients with HAE, in considering no attack triggering effect and a high safety profile. Due to the importance of the vaccination in control of the pandemic, providing well-informed education on vaccine efficacy, adverse effects, and the impact on HAE attacks could increase the rate of vaccination among these patients to control the COVID-19 pandemic. In patients with poor disease control, initiation of an LTP before vaccination can be considered to provide disease control and reduce the risk of attacks that may develop after vaccination.
Footnotes
The authors have no conflicts of interest to declare pertaining to this article
No external funding sources reported
REFERENCES
- 1. Maurer M, Magerl M, Betschel S, et al. The international WAO/EAACI guideline for the management of hereditary angioedema—The 2021 revision and update. Allergy. 2022; 77:1961–1990. [DOI] [PubMed] [Google Scholar]
- 2. Caballero T, Maurer M, Longhurst H, et al. Triggers and prodromal symptoms of angioedema attacks in patients with hereditary angioedema. J Investig Allergol Clin Immunol. 2016; 26:383–386. [DOI] [PubMed] [Google Scholar]
- 3. Zotter Z, Csuka D, Szabó E, et al. The influence of trigger factors on hereditary angioedema due to C1-inhibitor deficiency. Orphanet J Rare Dis. 2014; 9:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ghebrehiwet B, Kaplan AP, Joseph K, et al. The complement and contact activation systems: partnership in pathogenesis beyond angioedema. Immunol Rev. 2016; 274:281–289. [DOI] [PubMed] [Google Scholar]
- 5. Soleimanpour S, Yaghoubi A. COVID-19 vaccine: where are we now and where should we go? Expert Rev Vaccines. 2021; 20:23–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Banerji A, Wickner PG, Saff R, et al. mRNA vaccines to prevent COVID-19 disease and reported allergic reactions: current evidence and suggested approach. J Allergy Clin Immunol Pract. 2021; 9:1423–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Republic of Turkey, Ministry of Health. COVID-19 vaccine information platform. Available online at https://covid19asi.saglik.gov.tr/TR-77707/asi-uygulanacak-grup-siralamasi.html; accessed May 3, 2022.
- 8. Anand P, Stahel VP. Review the safety of Covid-19 mRNA vaccines: a review. Patient Saf Surg. 2021; 15:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Riad A, Sağıroğlu D, Üstün B, et al. Prevalence and risk factors of CoronaVac side effects: an independent cross-sectional study among healthcare workers in Turkey. J Clin Med. 2021; 10:2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fijen LM, Levi M, Cohn DM. COVID-19 vaccination and the risk of swellings in patients with hereditary angioedema. J Allergy Clin Immunol Pract. 2021; 9:4156–4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Awijen H, Zaied YB, Nguyen D. Covid-19 vaccination, fear and anxiety: evidence from Google search trends. Social Sci Med. 2022; 297:114820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bygum A, Fagerberg CR, Ponard D, et al. Mutational spectrum and phenotypes in Danish families with hereditary angioedema because of C1 inhibitor deficiency. Allergy. 2011; 66:76–84. [DOI] [PubMed] [Google Scholar]
- 13. Huskisson EC. Measurement of pain. Lancet. 1974; II:1127–1131. [DOI] [PubMed] [Google Scholar]
- 14. Kochhar S, Salmon DA. Planning for COVID-19 vaccines safety surveillance. Vaccine. 2020; 38:6194–6198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grivcheva-Panovska V, Craig TJ, Longhurst H, et al. Global perceptions of the current and future impacts of COVID-19 on hereditary angioedema management. Allergy Asthma Proc. 2022; 43:e1–e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Joshi A, Kaur M, Kaur R, et al. Predictors of COVID-19 vaccine acceptance, intention, and hesitancy: a scoping review. Front Public Health. 2021; 9:698111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bellanti JA. COVID-19 vaccines and vaccine hesitancy: role of the allergist/immunologist in promotion of vaccine acceptance. Allergy Asthma Proc. 2021; 42:386–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Republic of Turkey, Ministry of Health. COVID-19 vaccine information platform. Available online at https://covid19asi.saglik.gov.tr/; accessed May 3, 2022.
- 19. Dror AA, Eisenbach N, Taiber S, et al. Vaccine hesitancy: the next challenge in the fight against COVID-19. Eur J Epidemiol. 2020; 35:775–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lazarus JV, Wyka K, Rauh L, et al. Hesitant or not? The association of age, gender, and education with potential acceptance of a COVID-19 vaccine: a country-level analysis. J Health Commun. 2020; 25:799–807. [DOI] [PubMed] [Google Scholar]
- 21. Wang J, Jing R, Lai X, et al. Acceptance of COVID-19 vaccination during the COVID-19 pandemic in China. Vaccines (Basel). 2020; 8:482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Craig T. Triggers and short-term prophylaxis in patients with hereditary angioedema. Allergy Asthma Proc. 2020; 41(suppl 1):S30–S34. [DOI] [PubMed] [Google Scholar]
- 23. Can Bostan O, Tuncay G, Damadoglu E, et al. Effect of COVID-19 on hereditary angioedema activity and quality of life. Allergy Asthma Proc. 2021; 42:403–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Eyice Karabacak D, Demir S, Yeğit OO, et al. Impact of anxiety, stress and depression related to COVID-19 pandemic on the course of hereditary angioedema with C1-inhibitor deficiency. Allergy. 2021; 76:2535–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Menni C, Klaser K, May A, et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Infect Dis. 2021; 21:939–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020. Dec 31; 383:2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wu Z, Hu Y, Xu M, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy adults aged 60 years and older: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021; 21:803–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cabanillas B, Novak N. Allergy to COVID-19 vaccines: a current update. Allergol Int. 2021; 70:313–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ozturk A, Çağlayan B, Kapmaz M, et al. Hypersentivity reactions to COVID-19 vaccines: a case of eosinophilic pneumonia following Sinovac/CoronaVac vaccination. Eur Ann Allergy Clin Immunol. 2022; 1764-1489.247. [DOI] [PubMed] [Google Scholar]
- 30. Sokolowska M, Eiwegger T, Ollert M, et al. EAACI statement on the diagnosis, management and prevention of severe allergic reactions to COVID-19 vaccines. Allergy. 2021; 76:1629–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Squeglia V, Barbarino A, Bova M, et al. High attack frequency in patients with angioedema due to C1-inhibitor deficiency is a major determinant in switching to home therapy: a real-life observational study. Orphanet J Rare Dis. 2016; 11:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Demir S, Ünal D, Olgaç M, et al. Evaluating adherence to long-term prophylaxis treatment with danazol in adult hereditary angioedema patients: areal life study. Marmara Medical J. 2019; 32:7–13. [Google Scholar]