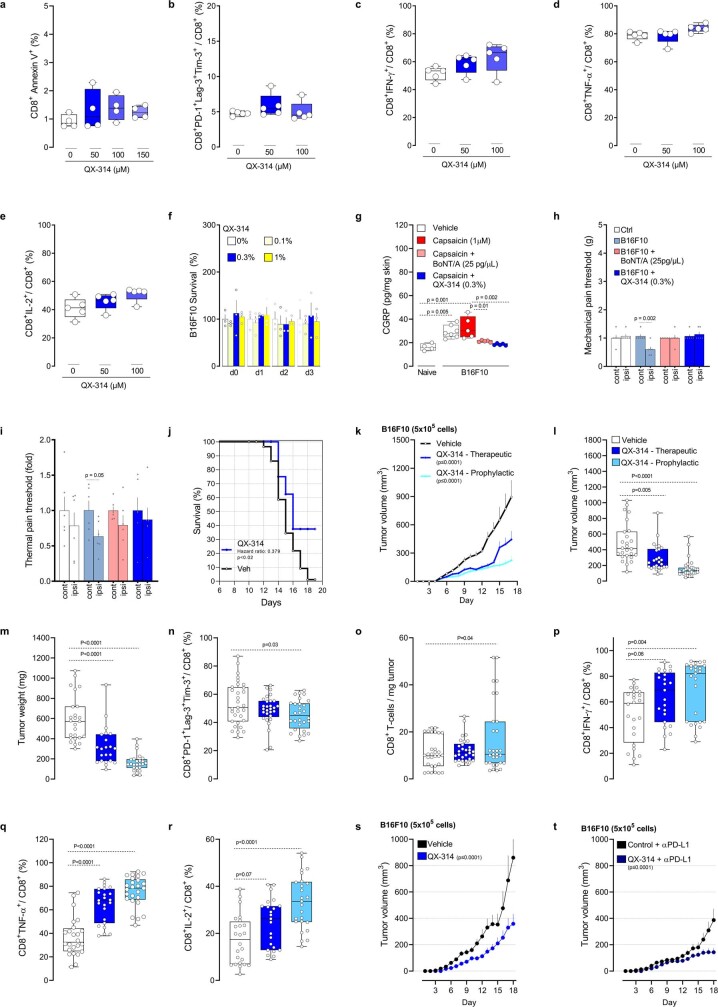
Extended Data Fig. 8. QX-314 silencing of B16F10-innervating neurons reduces tumour growth.
(a–e) Splenocytes-isolated CD8+ T cells from naive C57BL6J mice were cultured under Tc1-stimulating conditions (ex vivo activated by CD3 and CD28, IL-12, and anti-IL4) for 48h. The cells were then exposed to QX-314 (50–150 μM) for 24h, effects on apoptosis, exhaustion and activation were measured by flow cytometry. When compared to vehicle-exposed cells, QX-314 did not affect the survival of cultured cytotoxic CD8+ T cells (a), nor their relative expression of PD-1+LAG3+TIM3+ (b), IFNγ+ (c), TNF+ (d) and IL-2+ (e). (f) B16F10 (1x105 cells) were cultured for 24h. The cells were then exposed or not to QX-314 (0.1-1%) for an additional 24-72h, and cell count was analysed by bright-field microscopy. QX-314 did not affect B16F10 cells’ survival, as measured by relative cell count changes (at each time point) in comparison to vehicle-exposed cells. (g–i) One and three days prior to tumour inoculation, 8-week-old male and female wild-type mice’s right hindpaws or flanks were injected with BoNT/A (25 pg/μL; i.d.) or its vehicle. On the following day, orthotopic B16F10 cells (g: 5x105 cells; i.d.; h–i: 2x105 cells; i.d.) were inoculated into the area pre-exposed to BoNT/A. Starting one day post inoculation, QX-314 (0.3%) or its vehicle was administered (i.d.) once daily in another group of mice. The effects of sensory neuron silencing were tested on neuropeptide release (g), as well as mechanical (h) and thermal pain hypersensitivity (i). First, CGRP levels were increased in B16F10 tumour surrounding skin explant (assessed on day 15) in comparison to control skin; an effect further enhanced by capsaicin (1 μM; 3h) but was absent in skin pre-treated with BoNT/A (25 pg/μL) or QX-314 (0.3%; g). We also found that B16F10 injection induced mechanical (h) and thermal pain hypersensitivities (i) fourteen days post tumour inoculation. These effects were stopped by sensory neuron silencing with QX-314 or BoNT/A (h–i). (j) Orthotopic B16F10-mCherry-OVA cells (5x105 cells; i.d.) were inoculated into 8-week-old male and female mice. Starting one day post inoculation, QX-314 (0.3%; i.d.; 5 sites) was injected once daily around the tumour. The effect of nociceptor neuron silencing on tumour size and tumour-infiltrating CD8+ T cell exhaustion was measured. We found that silencing tumour-innervating neurons increased the mice’s median length of survival (~270% Mantel–Haenszel hazard ratio; measured on day 19). (k–r) Orthotopic B16F10-mCherry-OVA cells (5x105 cells; i.d.) were inoculated into 8-week-old male and female mice. Starting one day post inoculation (defined as prophylactic), In other groups of mice, QX-314 daily injection started once the tumour reached a volume of ~200mm3 (defined as therapeutic). As measured seventeen days post tumour inoculation, silencing tumour innervation also decreased tumour volume (k,l) and weight (m), as well as the relative proportion of PD-1+LAG3+TIM3+ (n) CD8+ T cells. QX-314 treatment also increased the total number of intratumoral CD8+ T cells (o), as well as relative proportion of IFNγ+ (p), TNF+ (q), and IL-2+ (r) CD8+ T cells. (s–t) Orthotropic B16F10-mCherry-OVA cells (5x105 cells, i.d.) were injected into mice treated with QX-314 (0.3%; i.d.) 2-3 days prior to being injected into vehicle-exposed mice. Mice from each group with similar tumour size (~100mm3) were selected and exposed to αPD-L1 (6 mg/kg, i.p.) once every 3 days for a total of 9 days. Eighteen days post tumour inoculation, we found that αPD-L1-reduced tumour growth was higher (~61%) in nociceptor silenced mice than was observed in isotype vehicle-exposed mice (~49%; s-t). Data are shown as box-and-whisker plots (runs from minimal to maximal values; the box extends from 25th to 75th percentile and the middle line indicates the median), for which individual data points are given (a–e, g, l–r), as mean ± S.E.M (f,h,i,k,s,t), or as Mantel–Cox regression analysis (j). N are as follows: a: n = 4/groups, b–e: n = 5/groups, f: n = 3/groups, g: naïve (n = 4), vehicle (n = 7), B16F10+vehicle (n = 5), B16F10+BoNT/A (n = 5), B16F10+QX-314 (n = 5), h–i: n = 6/groups, j: vehicle (n = 89), QX-314 (n = 12), k: vehicle (n = 21), QX-314 prophylactic (n = 21), QX-314 therapeutic (n = 17), l: vehicle (n = 26), QX-314 therapeutic (n = 26), QX-314 prophylactic (n = 28), m: vehicle (n = 25), QX-314 therapeutic (n = 22), QX-314 prophylactic (n = 25), n: vehicle (n = 31), QX-314 therapeutic (n = 29), QX-314 prophylactic (n = 28), o: n = 30/groups, p–r: vehicle (n = 24), QX-314 therapeutic (n = 23), QX-314 prophylactic (n = 25), s: vehicle (n = 9), QX-314 (n = 13), t: vehicle + αPD-L1 (n = 18), QX-314 + αPLD1 (n = 13). Experiments were independently repeated two (a–i, s–t) or four (j–r) times with similar results. P-values are shown in the figure and determined by one-way ANOVA posthoc Bonferonni (a–g, l–r), two-sided unpaired Student’s t-test (h–i), Mantel–Cox regression (j), or two-way ANOVA posthoc Bonferroni (k, s–t).