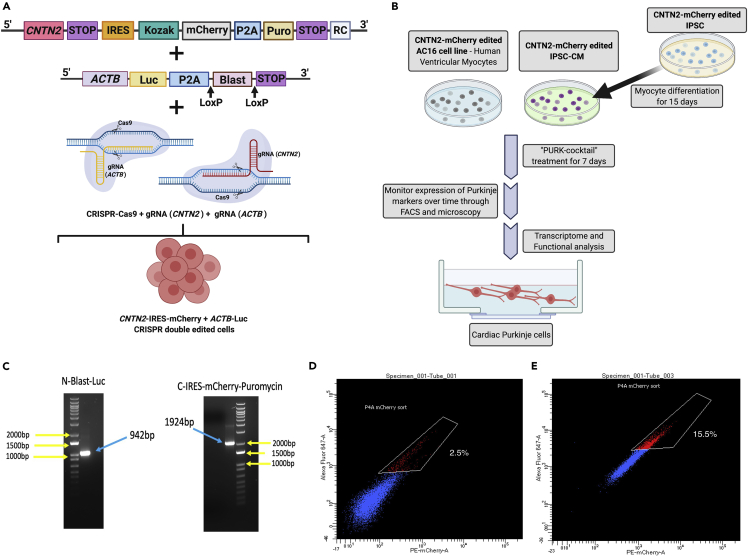
Figure 1.
Strategy for creating Purkinje-like cells
(A) Co-gene edition approach using CRISPR-Cas9 knock-in system, where a mCherry-IRES-Puromycin tag is added at the c-terminal of CNTN2 gene at the same time that a Blasticidin-Luciferase (Blast-Luc) tag is added to the ACTB gene to allow the selection of the gene-edited cells prior to differentiation, and after Purkinje differentiation.
(B) Overall scheme for differentiating CMs into cardiac Purkinje-like cells using our small molecule cocktail (“PURK-cocktail”). In brief, the AC16-CM and iPSC were co-gene edited using our CRISPR-Cas9 Knock-in approach. The iPSCs were differentiated into myocytes (iPSC-CM). The AC16-CM and iPSC-CM were then treated with the PURK-cocktail and characterized by downstream transcriptome and functional analysis to determine if the differentiated cells were cardiac Purkinje-like.
(C) PCR genotyping confirms the cells were successfully gene-edited using CRISPR-Cas9 at the CTNT2 and ACTB genes.
(D and E) (D) FACS analysis of control (2.5 ± 0.9%) and (E) PURK-cocktail-treated cells (15.5 ± 1.2%) showed a significant difference in the amount of CNTN2-mCherry + cells (p < 0.005). The control (vehicle)-treated cell population showed minimal expression of CNTN2-IRES-mCherry + cells, whereas the PURK-cocktail-treated cells showed a significant amount of CNTN2-IRES-mCherry + cell population. Created using BioRender.com.