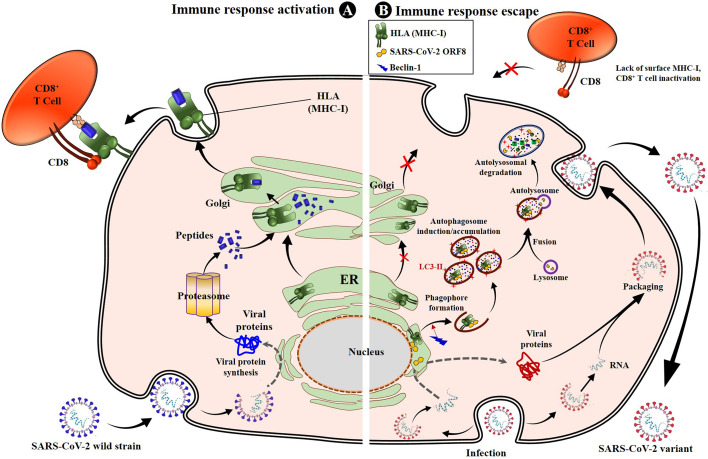
Figure 1.
SARS-CoV-2 impairs antigen presentation by MHC-I to CD8+ T cells through ORF8. Once entered into the epithelial cells by endocytosis, the genomic RNA is released. SARS-CoV-2 uses the cell protein expression machinery to synthesize the viral proteins. The antigen processing and presentation pathways occur for viral protein lysis into peptides. Simultaneously, the MHC-I will mature in the ER and migrate to the Golgi apparatus, where peptides will be loaded onto MHC-I molecules and presented to CD8+ cytotoxic T lymphocytes (CTLs), activating the cell cytotoxic response (A). When infected with SARS-CoV-2, especially variants of concern, the viral proteins, including the ORF8, are produced inside ER-derived DMVs containing LC3-I. The synthesized ORF8 protein directly interacts with the MHC-I and leads MHC-I trafficking from ER to autophagosome vesicles, inducing the early stages of autophagy and accumulation of autophagosomes thanks to beclin 1-activated upregulation. The matured autophagosome then fuses with the lysosome to form the autolysosome, inside which MHC-I is digested by lysozymes. This results in the loss of sensitivity of SARS-CoV-2–infected cells to CD8+ T cells and lysis by CTLs. When infection occurs with VOCs, the mechanism is strongly enhanced, and the SARS-CoV-2 variant easily escapes T cells (B). ER, endoplasmic reticulum; DMV, doubled membrane vesicle; LC3-I, microtubule-associated protein 1A/1B-light chain 3B; MHC-I, major histocompatibility complex type I.