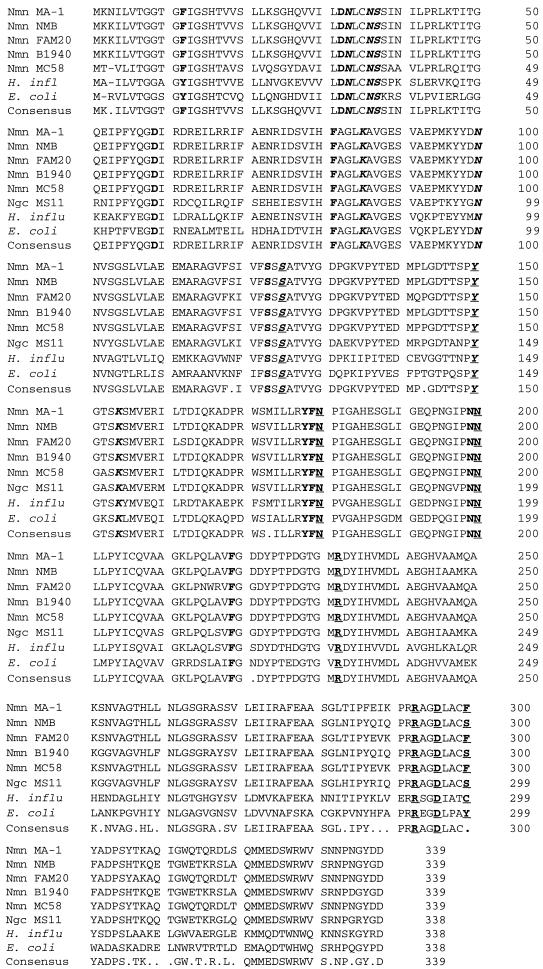
FIG. 7.
Predicted amino acid alignment of GalE proteins from various gram-negative bacteria. The consensus sequence is shown at the bottom. The meningococcal polypeptide is 1 amino acid longer than the protein from gonococci, Haemophilus influenzae, or E. coli. According to the structural data from the E. coli epimerase (3, 17, 26, 28), the amino acids that form the binding pockets of NAD+ and UDP-sugar are boldfaced. The amino acids that form hydrogen bonds with NAD+ are italicized, and those bonding with UDP-sugar are underlined. The residues involved in binding of both the cofactor and the substrate are italicized and underlined. S124 and Y149 of the E. coli protein are critical for catalysis, and these residues are conserved in both prokaryotes and eukaryotes. Nmn, N. meningitidis, Ngc, Neisseria gonorrhoeae; H. influ, H. influenzae.