Abstract
Background
In Ayurveda; an Indian system of traditional medicine, Ocimum sanctum is said to have remedial effect on hriddaurbalya (problems affecting the mind), aakshepayukta vikara (nervous disorders) and shiroroga (diseases of head). Hence, in Ayurvedic practice, it is profoundly used as an antistress medicine. Stress is known to affect neurons of functionally significant brain regions like substantia nigra. However, experimental evidence showing its effect on morphology of substantia nigral neurons is lacking. In addition, whether the O. sanctum treatment attenuates stress induced substantia nigral neuronal structural changes is not known.
Objectives
To know the effect of stress on morphology of substantia nigral neurons and the effect of O. sanctum fresh leaf extract (OSE) on substantia nigral neurons of stressed rats.
Material and methods
Present study included three experiments. Experiment I: To study the effect of 3 and 6 weeks of foot shock stress in rats; Experiment II- To study the effect of 3 weeks of OSE treatment on 3 week-stress undergoing rats and on 3 week-stressed rats; Experiment III- To study the effect of 6 weeks of OSE treatment in 6 week-stress undergoing rats and in 6 week-stressed rats.
Results
In experiment I, stress had significant deleterious effect on dendritic arborization of substantia nigral neurons. Experiments II and III showed prevention and attenuation of the stress induced dendritic atrophy of substantia nigral neurons in both 2 ml and 4 ml OSE treatment groups. Protective effect of OSE was more pronounced in rats which are treated for a longer duration.
Conclusions
Foot shock stress induces neuronal damage in the substantia nigra of rats. Treatment with fresh leaf extract of O. sanctum could prevent and attenuate the foot shock stress induced behavioral deficit and substantia nigral neuronal damage.
Keywords: Dendritic branching, Dendritic intersections, Open field test, Foot shock stress, Herbal extract, Stress behavior
1. Introduction
Stress has been reported to affect the brain and behavior of humans and experimental animals of all ages. Learning and memory impairments have been observed in animals undergoing stress. Stress is known to affect neurons of brain regions like hippocampus, substantia nigra, amygdala and cerebral cortex. Stress is known to impair non-social behavior in male rats such as decrease in motor activity [1] and reduced motivation to explore the environment [2]. Many laboratory models of stress have been used in assessing the various mechanisms of stress-related disorders and to study the effect of various drugs on these disorders [3].
Substantia nigra is situated in the ventral part of the tegmentum of midbrain. Among the neurons of substantia nigra, large neurons in pars compactum are considered to be predominantly dopaminergic and functionally very relevant [4]. Stress induced dopamine dysfunction has been well documented [5]. There are reports of stress and depression causing substantia nigral destruction leading to Parkinson's disease [6,7]. Further, it has been reported that foot shock stress causes consistent extensive hypersensitivity and anhedonic behavior, psychological stress and anxiety [8,9]. Hence, in the present study, foot shock stress and neurons of pars compacta of substantia nigra have been considered.
Estimation of dendritic intersections and dendritic branches is known to give an indication of length and arborization of neuronal dendrites respectively. Any increase in these measures, reflects increased functional efficacy of neurons [10]. Considering these facts, estimation of dendritic morphology of substantia nigral neurons has been done in the present study to assess the stress induced morphological changes.
Ocimum sanctum has an important place in traditional as well as modern pharmacological systems of medicine. The Indian Materia Medica refers to O. sanctum as a plant having medicinal properties of very high value [11]. Traditionally, O. sanctum is believed to have curative effect on hriddaurbalya. Hridaya is said to be the seat of mind as per Ayurveda. Anything that affects hridaya will have an influence on mind. Hence, “hriddaurbalya” can be understood as stress/depression/mental weakness/structural and functional derangement of heart. It is also curative for aakshepayukta vikara (nervous system related disorders causing contractures or seizures) and shiroroga (diseases of head) [12]. There are reports of increase in survival time of swimming and prevention of stress-induced ulcers when stressed rats were treated with O. sanctum [13]. Stress-induced gastric ulcers improved partially by pretreatment with O. sanctum [14]. Pretreatment with O. sanctum prevented stress-induced decrease in levels of adrenaline, noradrenaline and monoamine oxidase enzyme and increase in levels of serotonin in brain [15]. O. sanctum reduced stress-induced delayed entry of rats into restrainers and struggle inside restrainers [16]. It also reduced the incidence of gastric ulcer formation in swimming endurance test in albino rats [17].
In Ayurvedic literature, though there are mentions of antistress effect of O. sanctum, its effect on morphology of neurons of brain regions such as substantia nigra is not reported. In addition, most of the studies are conducted with its various extracts, but, not with fresh leaf juice. Leaf juice is the most easily available and also commonly recommended effective dosage form by Ayurveda. Moreover, not many reports are available on induction of foot shock stress using serial uniform electric grid. We hypothesize that treatment with fresh leaf juice of O. sanctum will attenuate the structural changes in substantia nigral neurons of foot shock stress induced rats. Hence, the objective of the present study was to study the effect of O. sanctum fresh leaf extract (OSE) on substantia nigral neurons of the stressed rats.
2. Materials and methods
Experiments were conducted on young adult (two and half month old) Wistar rats of both genders. Rats were bred and maintained in central animal house, of the University and all experiments were carried out with prior approval from the institutional animal ethical committee (IAEC/KMC/2002-2003 dated 04 March 2003).
Experimental design: Whole study was conducted under 3 experiments. Detailed experimental design is shown in Fig. 1.
Fig. 1.
Flow charts showing the experimental designs of 1A-Experiment I (stress groups); 1B- Experiment II (stress + 3 weeks OSE treatment groups) and Experiment III (stress + 6 weeks OSE treatment groups).
Time schedule of the experiment: Selected rats were assigned to the corresponding groups. Stress groups were subjected to foot shock stress for 3 h/day for 3 or 6 weeks depending on the group. The rats were treated with 2 ml or 4 ml of OSE/kg/day for 3 or 6 weeks either during the period of exposure to stress or following the exposure to the stress depending on the group. Rats were then subjected to open field tests following which they were sacrificed. Body weight was monitored throughout experimental period.
2.1. Electric foot shock stress
Rats were stressed by giving intermittent electric foot shock for a given period in an electric foot shock apparatus. The apparatus was designed to provide electric shock at the Voltage range of 0–150 V with the frequency of 50 Hz using AC current. The maximum current output which could be provided by the apparatus was 500 mA. Electric foot shock timer was set to generate the shock at 5 min interval for 3 s (i.e. 12 foot shocks/hour). Rats were placed individually in closed (with adequate ventilation) shock grid compartments. Foot shock was given for 3 h daily for different duration as per the experimental design. In this apparatus, multiple animals could uniformly be stressed simultaneously and no animal could escape the foot shock stress.
2.2. Body weight gain
The rats were weighed before the commencement of experiments and also at the end of experiments to note the initial and final body weights and weight gained by rats during the experiment was calculated.
2.3. Fresh leaf juice extraction
Fresh juice from the O. sanctum leaves was extracted using Pidana and Vastra Putam method of swarasa preparation. Fresh and mature O. sanctum plant leaves (8-10 from the growing tip excluding 4 leaves at the tip) (Fig. 2) were collected daily. Leaves were washed, air dried for 30 min and homogenized. Juice so obtained was filtered using a clean piece of cloth. A known volume (1.54 ± 0.08 ml) of juice was extracted from a given weight (5.0 g) of leaves. Since uniform soil and water conditions were maintained throughout, we could extract same volume of juice from given weight of leaves on different days. Further, we have established that, the dry weight of a given volume (1 ml) of juice prepared on different days is same (45 ± 0.0005 mg, n = 6 samples).
Fig. 2.
Photograph showing fresh leaves of Ocimum sanctum.
In the present study, enrich fractionated extraction protocols have been avoided as they involve boiling the leaves with water or ethyl alcohol or other organic solvents which may alter the structure of bioactive principles. Though there may be minor variation in daily preparations, it will be minimal as leaves of equal maturation were collected from same place on all days and by long period (6 weeks) of treatment [18].
O. sanctum plants used in the present study were authenticated by Professor Venugopal Tantry, Formerly Professor of Botany at Vijaya College, Mulky, Karnataka, India. A voucher specimen (No.pp 531) has been maintained at the Department of Pharmacognosy, Manipal College of Pharmaceutical Sciences, Manipal Academy of Higher Education, Manipal, India.
Plant extract was administered orally, using a capillary tube attached to a tuberculin syringe as per the experimental design (Fig. 1).
2.4. Behavioral performance- open field test
Open field test is one of the most commonly used behavioral tests in rats to measure their behaviors ranging from overall locomotor activity to anxiety-related emotional behaviors [19]. As intact substantia nigra is vital for normal locomotory and emotional behavior, following the stress or saline/OSE treatment, rats were subjected to open field behavioral tests to assess the effect of foot shock stress on their locomotor performance. During the 5-min test, total number of peripheral and central square entries by rat were recorded. After each test, animal was returned to its home cage and number of boli of excreta was counted. After each test, floor was thoroughly cleaned and tests were repeated for 5 times for each rat.
2.5. Observation of gastric ulcers
Following behavioral tests, rats were deeply anesthetized with ether, abdomen was opened, stomach was opened along its greater curvature and gastric mucosa was observed under dissection microscope for the presence of ulcers.
2.6. Dendritic quantification of substantia nigral neurons [10,20,21]
Following the behavioral tests, the rats were deeply anesthetized and the brain was removed and fixed in rapid Golgi fixative. Tissue was processed for rapid Golgi staining, 120 μ thick sections were taken, and mounted on a slide. From each rat, 8 to 10 neurons from the substantia nigra compactum were traced using camera lucida and their dendritic intersections, branching points and processes were quantified. Concentric circle method of Sholl was used for dendritic quantification as reported earlier [[10], [20], [21]].
2.7. Data analysis
Data was analyzed using Student's t-test and analysis of variance (ANOVA) followed by Bonferroni's post-test using Graph Pad in Stat (GPIS) software, version 1.13. P values less than or equivalent to 0.05 were considered as significant.
3. Results
3.1. Experiment I- effect of 3 and 6 weeks of foot shock stress on rats
Body weight and gastric ulcer: There was significant (P < 0.001) decrease in the body weight of rats of 3 and 6 week stressed groups when compared to corresponding normal controls. In addition, both the stressed groups were positive for the gastric ulcers.
Behavioral performance- Open field test: Three week stressed rats had a significantly high ambulation compared to normal rats. Significantly (P < 0.05) more number of central squares were entered by 3 week-stressed rats than normal rats. Six week stressed animals showed significantly high ambulation and increased (P < 0.01) number of central square entries when compared to normal control rats. The time spent in the peripheral zone by both the stressed group rats was more compared to that of corresponding NC groups indicating the stress induced anxiety in these animals.
Dendritic arborization of substantia nigral neurons: Both 3 and 6 week-stressed rats showed a significant (P < 0.05, P < 0.01 & P < 0.001) reduction in dendritic intersections at different concentric circles when compared to respective normal control groups. There was a significant reduction in branching points at 60-80 and 80-100μ concentric zones in 3 week-stressed group and at 20-40 and 60-80 zones (P < 0.01) in 6 week stressed rats when compared to that of respective normal control groups. Significant (P < 0.05 & P < 0.01) reduction in total number of dendritic branching points is seen in both stress groups when compared to that of respective normal control groups. Total number of dendritic processes arising from soma was significantly (P < 0.001) reduced only in 3 week-stressed rats when compared to that of normal control group.
3.2. Experiment IIA– effect of 3 weeks of O. sanctum treatment on 3-week stress-undergoing rats
Body weight and gastric ulcer: There was significant (P < 0.01) decrease in the body weight of rats of stress + saline group when compared to the normal control group. In addition, stress + saline group was positive for the gastric ulcers. However, there was no significant difference in body weight gain between NC and stress + OSE treated groups (Table 1A).
Table 1A.
Effect of 3 weeks of Ocimum sanctum treatment on 3 week-stress undergoing rats- Body weight gain, gastric ulcers and open field test performance.
| Groups | n | Body weight gain in grams | Gastric ulcers | Open field test |
||
|---|---|---|---|---|---|---|
| Peripheral squares entered | Central squares entered | No. of boli of excreta | ||||
| Normal Control (NC) | 6 | 38.5 ± 14.25 |
- ve | 79.0 ± 11.6 |
4.0 ± 1.63 |
2.57 ± 2.14 |
| Stress + Saline (S + S) | 6 |
17.0 ∗∗ ± 5.83 |
+ ve | 77.85 ± 25.91 |
4.71 ± 2.49 |
0.57 ± 0.97 |
| Stress + 2 ml OSE (S+2-OSE) | 6 | 30.85 ± 11.56 |
- ve | 80.28 ± 18.05 |
5.57 ± 1.71 |
1.14 ± 2.26 |
| Stress + 4 ml OSE (S+4-OSE) | 6 | 27.75 ± 15.57 |
- ve | 86.87 ± 18.85 |
3.12 ± 2.94 |
1.75 ± 2.18 |
| F value | 3.71 | 0.33 | 1.53 | 1.32 | ||
| P value | P < 0.05 | NS | NS | NS | ||
Each value represents Mean ± SD; NS-Not significant; -ve– Gastric ulcers Absent, + ve – Gastric ulcers Present, NC vs. S + S: ∗∗P < 0.01; No significant difference is found between the different groups in open field test (One way ANOVA, Bonferroni's test).
Behavioral performance - Open field test: Saline treated stress undergoing rats did not show any significant difference in ambulation, number of peripheral square entries, number of central square entries and number of boli of excreta compared to normal and OSE treated stress undergoing rats (Table 1A).
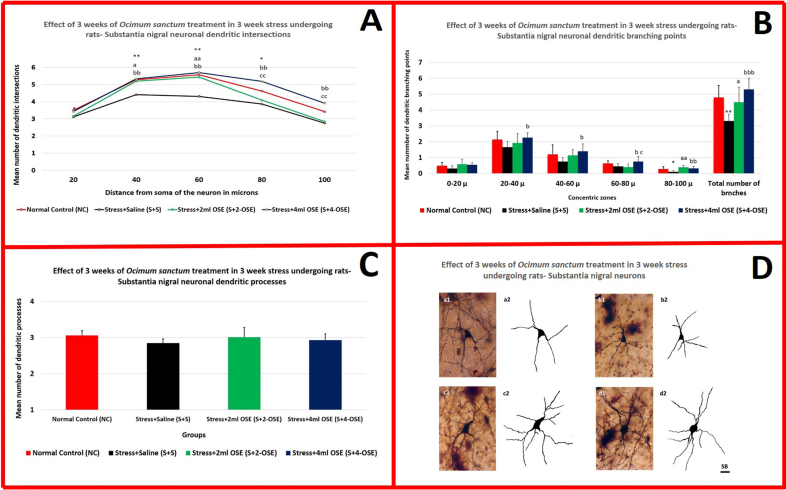
Dendritic arborization of substantia nigral neurons: In saline treated rats, dendritic intersections were significantly (P < 0.05 & P < 0.01) decreased at certain concentric circles compared to normal control and stress + OSE treated rats. Stress+4 ml OSE treated rats showed a significant (P < 0.05 & P < 0.01) increase in dendritic intersections in most of the concentric circles when compared to stress+2 ml OSE treated rats (Table 1B; Fig. 3A). Dendritic branching points were significantly decreased in saline treated stress undergoing rats at 80-100μ concentric zone (P < 0.05) compared to normal and stress+2 ml OSE treated groups. Saline treated rats showed a significant (P < 0.05 & P < 0.01) decrease in branching points at all zones except 0-20μ zone compared to that of stress+4 ml OSE treated rats. Compared to stress+2 ml OSE treated rats, the stress+4 ml OSE treated rats had significantly (P < 0.01) more number of dendritic branching points at 60-80μ zone. Similarly, saline treated stress undergoing rats showed a significant (P < 0.05, P < 0.01 & P < 0.001) reduction in total number of dendritic branching points compared to normal control and stress + OSE treated stress undergoing rats (Table 1C; Fig. 3B). However, no significant decrease in total number of dendritic processes arising from the soma was observed in saline treated stressed animals compared to normal control and stress + OSE treated animals (Table 1B; Fig. 3C). Further, there was no significant difference between NC and stress + OSE treated groups in any of the dendritic arborization parameters (Table 1B, Table 1C; Fig. 3A, B, Fig. 3C) Representative photomicrographs of silver impregnated substantia nigral neurons and their camera lucida tracings are shown in Fig. 3D.
Table 1B.
Effect of 3 weeks of Ocimum sanctum treatment on 3 week-stress undergoing rats- Substantia nigral neuronal dendritic intersections and processes.
| Groups | n | Distance from Soma (μ) |
No. of dendritic processes | ||||
|---|---|---|---|---|---|---|---|
| 20 | 40 | 60 | 80 | 100 | |||
| Normal Control (NC) | 6 | 3.5 ± 0.36 |
5.28 ± 0.56 |
5.57 ± 0.54 |
4.62 ± 0.56 |
3.42 ± 0.62 |
3.06 ± 0.13 |
| Stress + Saline (S + S) | 6 |
3.11 ∗ ± 0.17 |
4.41 ∗∗ ± 0.38 |
4.31 ∗∗ ± 0.34 |
3.86 ∗ ± 0.46 |
2.75 ± 0.35 |
2.85 ± 0.12 |
| Stress+2 ml OSE (S+2-OSE) | 6 | 3.16 ± 0.3 |
5.19 a ± 0.47 |
5.43 aa ± 0.68 |
4.09 ± 0.61 |
2.84 ± 0.58 |
3.01 ± 0.27 |
| Stress+4 ml OSE (S+4-OSE) | 6 | 3.43 ± 0.23 |
5.33 bb ± 0.32 |
5.7 bb ± 0.56 |
5.18 bb cc ± 0.61 |
3.91 bb cc ± 0.64 |
2.93 ± 0.17 |
| F value | 2.9887 | 5.7388 | 8.2547 | 6.5471 | 5.6253 | 1.5308 | |
| P value | P < 0.05 | P < 0.01 | P < 0.001 | P < 0.01 | P < 0.01 | NS | |
Each value represents Mean ± SD; n = number of rats, NS – not significant, NC vs. S + S: ∗P < 0.05, ∗∗P < 0.01; S + S vs. S+2-OSE: a P < 0.05, aa P < 0.01; S + S vs. S+4-OSE: bb P < 0.01; S+2-OSE vs. S+4-OSE: cc P < 0.01; (One way ANOVA, Bonferroni's test).
Fig. 3.
3A- Dendritic intersections; 3B- Dendritic branching points; 3C- Dendritic processes: Each point represents mean of 8–10 neurons from each rat. NC vs. S + S: ∗P < 0.05, ∗∗P < 0.01; S + S vs. S+2-OSE: a P < 0.05, aa P < 0.01; S + S vs. S+4-OSE: b P < 0.05, bb P < 0.01, bbb P < 0.001; S+2-OSE vs. S+4-OSE: c P < 0.05, cc P < 0.01 (One way ANOVA, Bonferroni's test). 3D- Substantia nigral neurons: Representative photomicrographs of silver impregnated substantia nigral neurons and camera lucida tracings from normal control rats (a1, a2), stress + saline treated rats (b1, b2), stress+2-OSE treated rats (c1, c2) and stress+4-OSE treated rats (d1, d2). A significant decrease in the dendritic arborization can be noted in stress + saline treated rats. However, dendritic arborization of stress+2-OSE and stress+4-OSE treated rats look at par with that of normal control rats. Scale bar (SB)-20μm.
Table 1C.
Effect of 3 weeks of Ocimum sanctum treatment on 3 week-stress undergoing rats- Substantia nigral neuronal dendritic branching points.
| Groups | n | Concentric zones |
Total dendritic branching points | ||||
|---|---|---|---|---|---|---|---|
| 0-20 μ | 20-40 μ | 40-60 μ | 60-80 μ | 80-100 μ | |||
| Normal Control (NC) | 6 | 0.49 ± 0.21 |
2.14 ± 0.52 |
1.21 ± 0.6 |
0.64 ± 0.16 |
0.29 ± 0.15 |
4.79 ± 0.76 |
| Stress + Saline (S + S) | 6 | 0.31 ± 0.18 |
1.66 ± 0.36 |
0.75 ± 0.25 |
0.46 ± 0.16 |
0.11 ∗ ± 0.075 |
3.31 ∗∗ ± 0.43 |
| Stress+2 ml OSE (S+2-OSE) | 6 | 0.59 ± 0.32 |
1.92 ± 0.6 |
1.15 ± 0.36 |
0.4 ± 0.2 |
0.39 aa ± 0.12 |
4.49 a ± 0.94 |
| Stress+4 ml OSE (S+4-OSE) | 6 | 0.55 ± 0.13 |
2.26 b ± 0.3 |
1.4 b ± 0.46 |
0.75 b c ± 0.32 |
0.33 bb ± 0.12 |
5.3 bbb ± 0.71 |
| F value | 1.87 | 1.96 | 2.34 | 3.22 | 6.12 | 7.95 | |
| P value | NS | NS | NS | P < 0.5 | P < 0.01 | P < 0.01 | |
Each value represents Mean ± SD; n = number of rats, NS – not significant, NC vs. S + S: ∗P < 0.05, ∗∗P < 0.01; S + S vs. S+2-OSE: a P < 0.05, aa P < 0.01; S + S vs. S+4-OSE: b P < 0.05, bb P < 0.01, bbb P < 0.001; S+2-OSE vs. S+4-OSE: c P < 0.05; (One way ANOVA, Bonferroni's test).
3.3. Experiment IIB- effect of 3 weeks of O. sanctum treatment on 3-week stressed rats
Body weight and gastric ulcer: There was decrease (not significant) in the body weight of rats of stress + saline group when compared to the normal control group. All the groups were negative for the gastric ulcers. There was no significant difference in body weight gain between NC and stress + OSE treated groups (Table 2A).
Table 2A.
Effect of 3 weeks of Ocimum sanctum treatment on 3 week-stressed rats- Body weight gain, gastric ulcers and Open field test performance.
| Groups | n | Body weight gain in grams | Gastric ulcers | Open field test |
|||
|---|---|---|---|---|---|---|---|
| Peripheral squares entered | Central squares entered | No. of boli of excreta | |||||
| Normal Control (NC) | 6 | 48.5 ± 16.92 |
- ve | 68.85 ± 17.23 |
3.0 ± 2.76 |
0.85 ± 1.46 |
|
| Stress + Saline (S + S) | 6 | 38.66 ± 17.11 |
- ve | 64.87 ± 27.28 |
3.25 ± 2.65 |
2.37 ± 2.55 |
|
| Stress+2 ml OSE (S+2-OSE) | 6 | 55.33 ± 17.03 |
- ve | 63.71 ± 21.48 |
2.14 ± 2.1 |
1.5 ± 0.5 |
|
| Stress+4 ml OSE (S+4-OSE) | 6 | 52.14 ± 7.69 |
- ve |
92.66 b c ± 17.37 |
6.33 #b c ± 2.8 |
1.83 ± 2.56 |
|
| F value | 1.3835 | 2.49 | 3.14 | 0.77 | |||
| P value | NS | NS | <0.05 | NS | |||
Each value represents Mean ± SD; NS- Not significant; - ve – Gastric ulcers absent. NC vs. S+4-OSE: #P < 0.05; S + S vs. S+4-OSE: b P < 0.05; S+2-OSE vs. S+4-OSE: c P < 0.05; No significant difference is found between the different groups in body weight gain (One way ANOVA, Bonferroni's test).
Behavioral performance -Open field test: Ambulatory movements were increased in stress+4 ml OSE treated rats. The stress+4 ml OSE treated rats showed a significant (P < 0.05) increase in number of peripheral square entries when compared with stress + saline and stress+2 ml OSE treated rats. They also showed significant (P < 0.05) increase in number of central square entries when compared with normal control, stress + saline and stress+2 ml OSE treated rats (Table 2A). Further, there was no significant difference between NC and stress+2 ml OSE treated rats in any of the behavioral parameters (Table 2A).
Dendritic arborization of substantia nigral neurons: Significant (P < 0.05, P < 0.01 & P < 0.001) decrease in dendritic intersections was observed in stress + saline treated rats in all the concentric circles compared to normal control rats. The stress + saline treated rats showed a significant (P < 0.001) decrease in intersections at 3 concentric circles compared to stress+2 ml OSE treated rats. These rats showed a significant (P < 0.01 & P < 0.001) decrease in intersections at all concentric circles except 100 μ when compared to stress+4 ml OSE treated rats. However, there was no significant difference in dendritic intersections between NC and stress + OSE treated rats (Table 2B). Stressed saline treated rats showed a significant (P < 0.05, P < 0.01 & P < 0.001) decrease in the dendritic branching points in certain concentric zones compared to normal control and stressed OSE treated rats. Similarly, a significant (P < 0.001 & P < 0.0001) reduction in total number of dendritic branching points was observed in the saline treated rats when compared to NC and OSE treated rats. Similarly, there was a significant (P < 0.01) increase in the dendritic branching points at 0-20 μ zone in both the stress + OSE treated groups when compared to that of NC group (Table 2C). Dendritic processes arising from the cell bodies were significantly (P < 0.05) reduced in saline treated stressed rats compared to normal control and stress+4 ml OSE treated rats (Table 2B).
Table 2B.
Effect of 3 weeks of Ocimum sanctum treatment on 3 week-stressed rats- Substantia nigral neuronal dendritic intersections and processes.
| Groups | n | Distance from soma (μ) |
No. of dendritic processes | ||||
|---|---|---|---|---|---|---|---|
| 20 | 40 | 60 | 80 | 100 | |||
| Normal Control (NC) | 6 | 3.76 ± 0.26 |
5.26 ± 0.46 |
5.65 ± 0.31 |
5.11 ± 0.56 |
4.03 ± 0.77 |
3.3 ± 0.3 |
| Stress + Saline (S + S) | 6 | 3.21 ∗∗ ± 0.28 |
4.31 ∗∗ ± 0.4 |
4.4 ∗∗∗ ± 0.25 |
3.85 ∗∗ ± 0.53 |
3.01 ∗ ± 0.53 |
2.9 ∗ ± 0.21 |
| Stress+2 ml OSE (S+2-OSE) | 6 | 4.13 aaa ± 0.29 |
5.58 aaa ± 0.67 |
5.65 aaa ± 0.39 |
4.61 ± 0.37 |
3.73 ± 0.77 |
3.21 ± 0.26 |
| Stress +4 ml OSE (S+4-OSE) | 6 | 4.08 bbb ± 0.39 |
5.23 bb ± 0.2 |
5.95 bbb ± 0.7 |
4.95 bb ± 0.84 |
3.83 ± 0.65 |
3.28 b ± 0.29 |
| F value | 11.23 | 8.33 | 14.25 | 5.24 | 2.51 | 2.89 | |
| P value | P < 0.001 | P < 0.001 | P < 0.0001 | P < 0.01 | NS | NS | |
Each value represents Mean ± SD; n = number of rats, NS – not significant, NC vs. S + S: ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001; S + S vs. S+2-OSE: aaa P < 0.001; S + S vs. S+4-OSE: b P < 0.05, bb P < 0.01, bbb P < 0.001; (One way ANOVA, Bonferroni's test).
Table 2C.
Effect of 3 weeks of Ocimum sanctum treatment in 3 week-stressed rats- Substantia nigral neuronal dendritic branching points.
| Groups | n | Concentric zones |
Total dendritic branching points | ||||
|---|---|---|---|---|---|---|---|
| 0-20 μ | 20-40 μ | 40-60 μ | 60-80 μ | 80-100 μ | |||
| Normal Control (NC) | 6 | 0.55 ± 0.2 |
2.5 ± 0.46 |
1.6 ± 0.53 |
0.96 ± 0.4 |
0.53 ± 0.39 |
6.15 ± 1.17 |
| Stress + Saline (S + S) | 6 | 0.36 ± 0.19 |
1.7 ∗∗ ± 0.33 |
0.83 ∗ ± 0.1 |
0.45 ∗ ± 0.37 |
0.18 ∗ ± 0.11 |
3.53 ∗∗∗ ± 0.41 |
| Stress +2 ml OSE (S+2-OSE) | 6 | 0.98 && aaa ± 0.18 |
2.48 aa ± 0.51 |
1.61 a ± 0.64 |
0.61 ± 0.38 |
0.38 ± 0.14 |
6.08 aaa ± 0.9 |
| Stress +4 ml OSE (S+4-OSE) | 6 | 1.0 ## bbb ± 0.32 |
2.61 bb ± 0.38 |
1.68 b ± 0.44 |
0.86 ± 0.32 |
0.33 ± 0.1 |
6.5 bbbb ± 0.46 |
| F value | 11.54 | 5.8 | 4.32 | 2.38 | 2.57 | 17.58 | |
| P value | P < 0.001 | P < 0.01 | P < 0.05 | NS | NS | <0.0001 | |
Each value represents Mean ± SD; n = number of rats, NS – not significant, NC vs. S + S: ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001; NC vs. S+2 OSE: && P < 0.01; NC vs. S+4 OSE: ##P < 0.01; S + S vs. S+2-OSE: a P < 0.05, aa P < 0.01, aaa P < 0.001; S + S vs. S+4-OSE: b P < 0.05, bb P < 0.01, bbb P < 0.001, bbbb P < 0.0001; (One way ANOVA, Bonferroni's test).
3.4. Experiment IIIA- effect of 6 weeks of O. sanctum treatment on 6-week stress-undergoing rats
Body weight and gastric ulcer: There was significant (P < 0.01, P < 0.05) decrease in the body weight of rats of S + S group when compared to the normal control and S+4-OSE groups. However, there was no significant difference in body weight gain between NC and OSE treated groups. In addition, S + S group was positive for the gastric ulcers (Table 3A).
Table 3A.
Effect of 6 weeks of Ocimum sanctum treatment on 6 week-stress undergoing rats- Body weight gain, gastric ulcers and open field test performance.
| Groups | n | Body weight gain in grams | Gastric ulcers | Open field test |
||
|---|---|---|---|---|---|---|
| Peripheral squares entered | Central squares entered | No. of boli of excreta | ||||
| Normal Control (NC) | 6 | 48.5 ± 16.92 |
- ve | 68.85 ± 17.23 |
3.0 ± 2.76 |
0.85 ± 1.46 |
| Stress + Saline (S + S) | 6 | 25.0 ∗∗ ± 8.28 |
+ ve | 88.14 ∗ ± 13.1 |
14.42 ∗∗∗∗ ± 1.61 |
1.0 ± 1.73 |
| Stress+2 ml OSE (S+2-OSE) | 6 | 38.0 ± 11.03 |
- ve | 73.28 ± 20.54 |
2.71 aaaa ± 2.21 |
2.14 ± 1.77 |
| Stress+4 ml OSE (S+4-OSE) | 6 | 40.0 b ± 16.63 |
- ve | 75.57 ± 23.55 |
2.0 bbbb ± 1.63 |
2.0 ± 2.0 |
| F value | 4.01 | 1.32 | 55.65 | 1.01 | ||
| P value | P < 0.05 | NS | P < 0.0001 | NS | ||
Each value represents Mean ± SD; NS- Not significant; - ve – Gastric ulcers Absent, + ve – Gastric ulcers present, NC vs. S + S: ∗∗P < 0.01, ∗∗∗∗P < 0.0001; S + S vs. S+2-OSE: aaaa P < 0.0001; S + S vs. S+4-OSE: b P < 0.05, bbbb P < 0.0001 (One way ANOVA, Bonferroni's test).
Behavioral performance - Open field test: Saline treated stress undergoing rats showed an increase in number of ambulations when compared to normal control and OSE treated stress undergoing rats. There was significant (P < 0.05) increase in peripheral square entries in stress + saline group when compared to that of normal control group. Number of central square entries during the test was significantly (P < 0.0001) increased in saline treated rats compared to normal control and both the stress + OSE treated groups. However, there was no significant difference in the peripheral square entries between the NC and stress + OSE treated groups indicating the normal behavior of stress + OSE treated rats (Table 3A).
Dendritic arborization of substantia nigral neurons: Dendritic intersections were significantly (P < 0.05, P < 0.01, P < 0.001 & P < 0.0001) decreased at all concentric circles in stress + saline treated rats compared to normal control and stress+2 ml or stress+4 ml OSE treated rats. The stress+2 ml OSE treated rats showed a significant (P < 0.05, P < 0.01) increase in dendritic intersections at 3 concentric circles when compared to normal control rats. The stress+4 ml OSE treated rats too had significantly (P < 0.05) increased dendritic intersections at outer 2 circles compared to normal control rats. However, stress+2 ml OSE treated rats had significantly (P < 0.01) more number of dendritic intersections at 20μ concentric circle compared to stress+4 ml OSE treated rats (Table 3B; Fig. 4A). Dendritic branching points were significantly (P < 0.05, P < 0.01, P < 0.001 & P < 0.0001) decreased in stress + saline treated group at certain concentric zones when compared to normal control and stress+2 ml or stress+4 ml OSE treated groups. However, there was no significant difference in dendritic branching points between NC and stress + OSE treated groups in any of the concentric zones. Total number of dendritic branching points was significantly (P < 0.0001) reduced in saline treated rats compared to normal control and stress + OSE treated rats. However, there was no significant difference in total number of dendritic branching points between NC and stress + OSE treated groups (Table 3C; Fig. 4B). Total number of dendritic processes arising from the cell bodies was significantly (P < 0.05 & P < 0.01) reduced in saline treated stress undergoing rats compared to normal control and stress + OSE treated rats. However, there was no significant difference in total number of dendritic processes between NC and stress + OSE treated groups (Table 3B; Fig. 4C). Representative photomicrographs of silver impregnated substantia nigral neurons and their camera lucida tracings are shown in Fig. 4D.
Table 3B.
Effect of 6 weeks of Ocimum sanctum treatment on 6 week-stress undergoing rats- Substantia nigral neuronal dendritic intersections and processes.
| Groups | n | Distance from soma (μ) |
No. of dendritic processes | ||||
|---|---|---|---|---|---|---|---|
| 20 | 40 | 60 | 80 | 100 | |||
| Normal Control (NC) | 6 | 3.76 ± 0.26 |
5.26 ± 0.46 |
5.65 ± 0.31 |
5.11 ± 0.56 |
4.03 ± 0.77 |
3.3 ± 0.3 |
| Stress + Saline (S + S) | 6 | 3.21 ∗ ± 0.24 |
4.01 ∗∗∗ ± 0.41 |
4.08 ∗∗∗∗ ± 0.29 |
3.7 ∗∗∗ ± 0.34 |
2.86 ∗ ± 0.48 |
2.96 ∗ ± 0.26 |
| Stress+2 ml OSE (S+2-OSE) | 6 | 4.16 & aaa ± 0.37 |
6.13 && aaa ± 0.25 |
6.3 & aaaa ± 0.59 |
5.51 aaaa ± 0.52 |
4.3 aa ± 0.46 |
3.4 aa ± 0.21 |
| Stress+4 ml OSE (S+4-OSE) | 6 | 3.6 b cc ± 0.32 |
5.66 bbb ± 0.5 |
6.15 bbbb ± 0.32 |
5.73 # bbbb ± 0.35 |
5.05 # bbb ± 0.84 |
3.45 bb ± 0.17 |
| F value | 10.18 | 28.65 | 39.23 | 24.27 | 11.39 | 5.06 | |
| P value | P < 0.001 | P < 0.0001 | P < 0.0001 | P < 0.0001 | P < 0.001 | P < 0.01 | |
Each value represents Mean ± SD; n = number of rats, NC vs. S + S: ∗P < 0.05, ∗∗∗P < 0.001, ∗∗∗∗P < 0.0001; NC vs. S+2-OSE: & P < 0.05, && P < 0.01; NC vs. S+4-OSE: #P < 0.05; S + S vs. S+2-OSE: aa P < 0.01, aaa P < 0.001, aaaa P < 0.0001; S + S vs. S+4-OSE: b P < 0.05, bb P < 0.01, bbb P < 0.001, bbbb P < 0.0001; S+2-OSE vs. S+4-OSE: cc P < 0.01; (One way ANOVA, Bonferroni's test).
Fig. 4.
4A- Dendritic intersections; 4B- Dendritic branching points; 4C- Dendritic processes: Each point represents mean of 8–10 neurons from each rat. NC vs. S + S: ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, ∗∗∗∗P < 0.0001; NC vs. S+2-OSE: & P < 0.05, && P < 0.01; NC vs. S+4-OSE: #P < 0.05; S + S vs. S+2-OSE: a P < 0.05, aa P < 0.01, aaa P < 0.001, aaaa P < 0.0001; S + S vs. S+4-OSE: b P < 0.05, bb P < 0.01, bbb P < 0.001, bbbb P < 0.0001; S+2-OSE vs. S+4-OSE: cc P < 0.01 (One way ANOVA, Bonferroni's test). 4D- Substantia nigral neurons: Representative photomicrographs of silver impregnated substantia nigral neurons and camera lucida tracings from normal control rats (a1, a2), stress + saline treated rats (b1, b2), stress+2-OSE treated rats (c1, c2) and stress+4-OSE treated rats (d1, d2). A significant decrease in the dendritic arborization can be noted in stress + saline treated rats. However, dendritic arborization of stress+2-OSE and stress+4-OSE treated rats look at par with that of normal control rats. Scale bar (SB)-20μm.
Table 3C.
Effect of 6 weeks of Ocimum sanctum treatment on 6 week-stress undergoing rats- Substantia nigral neuronal dendritic branching points.
| Groups | n | Concentric zones |
Total dendritic branching points | ||||
|---|---|---|---|---|---|---|---|
| 0-20 μ | 20-40 μ | 40-60 μ | 60-80 μ | 80-100 μ | |||
| Normal Control (NC) | 6 | 0.55 ± 0.2 |
2.5 ± 0.46 |
1.6 ± 0.53 |
0.96 ± 0.4 |
0.53 ± 0.39 |
6.15 ± 1.17 |
| Stress + Saline (S + S) | 6 | 0.31 ± 0.13 |
1.08 ∗∗∗∗ ± 0.24 |
0.98 ∗ ± 0.17 |
0.46 ∗∗ ± 0.17 |
0.13 ∗ ± 0.081 |
2.98 ∗∗∗∗ ± 0.34 |
| Stress+2 ml OSE (S+2-OSE) | 6 | 0.4 ± 0.28 |
2.53 aaaa ± 0.29 |
1.41 ± 0.23 |
0.9 aa ± 0.16 |
0.55 a ± 0.1 |
5.9 aaaa ± 0.48 |
| Stress+4 ml OSE (S+4-OSE) | 6 | 0.45 ± 0.18 |
2.38 bbb ± 0.51 |
1.58 b ± 0.46 |
0.93 bb ± 0.13 |
0.65 bb ± 0.31 |
6.0 bbbb ± 0.4 |
| F value | 1.63 | 19.06 | 3.46 | 5.78 | 4.77 | 29.64 | |
| P value | NS | P < 0.0001 | P < 0.05 | P < 0.01 | P < 0.05 | P < 0.0001 | |
Each value represents Mean ± SD; n = number of rats, NS – not significant, NC vs. S + S: ∗P < 0.05, ∗∗P < 0.01, ∗∗∗∗P < 0.0001; S + S vs. S+2-OSE: a P < 0.05, aa P < 0.01, aaaa P < 0.0001; S + S vs. S+4-OSE: b P < 0.05, bb P < 0.01, bbb P < 0.001, bbbb P < 0.0001; (One way ANOVA, Bonferroni's test).
3.5. Experiment IIIB- effect of 6 weeks of O. sanctum treatment on 6 week-stressed rats
Body weight and gastric ulcer: There was significant (P < 0.05) increase in the body weight of rats of stress+2 OSE & stress+4 OSE groups when compared to the stress + saline group rats. However, there was no significant difference in body weight gain between NC and stress + OSE treated groups. None of the groups were positive for gastric ulcers (Table 4A).
Table 4A.
Effect of 6 weeks of Ocimum sanctum treatment on 6 week-stressed rats- Body weight gain, gastric ulcers and open field test performance.
| Groups | n | Body weight gain in grams | Gastric ulcers | Open field test |
||
|---|---|---|---|---|---|---|
| Peripheral squares entered | Central squares entered | No. of boli of excreta | ||||
| Normal Control (NC) | 6 | 66.37 ± 18.53 |
- ve | 64.57 ± 13.37 |
3.0 ± 3.05 |
3.42 ± 2.81 |
| Stress + Saline (S + S) | 6 | 57.12 ± 16.14 |
- ve | 89.57 ∗ ± 16.25 |
12.85 ∗∗ ± 8.98 |
0.85 ± 2.26 |
| Stress+2 ml OSE (S+2-OSE) | 6 | 78.12 a ± 18.04 |
- ve | 66.12 a ± 21.64 |
2.75 aa ± 3.01 |
1.5 ± 2.5 |
| Stress+4 ml OSE (S+4-OSE) | 6 | 79.75 b ± 22.72 |
- ve | 59.85 b ± 20.38 |
4.28 bb ± 3.3 |
4.14 ± 3.62 |
| F value | 2.506 | 3.68 | 6.13 | 2.15 | ||
| P value | NS | P < 0.05 | P < 0.01 | NS | ||
Each value represents Mean ± SD; N- Not significant; - ve – Gastric ulcers Absent, NC vs. S + S: ∗P > 0.05, ∗∗P < 0.01; S + S vs. S+2-OSE: a P < 0.05, aa P < 0.01; S + S vs. S+4-OSE: b P < 0.05, bb P < 0.01 (One way ANOVA, Bonferroni's test).
Behavioral performance -Open field test: Ambulation was significantly increased in stress + saline treated rats. Stress + saline treated rats showed significant (P < 0.05) increase in number of peripheral square entries compared to normal control and both the stress + OSE treated rats. Significantly (P < 0.01) more number of central squares were entered by stress + saline treated rats compared to normal control and both the stress + OSE treated rats. However, there was no significant difference in the peripheral square entries between the NC and stress + OSE treated groups indicating the normal behavior of stress + OSE treated rats (Table 4A).
Dendritic arborization of substantia nigral neurons: There was a significant (P < 0.05, P < 0.01 & P < 0.001) decrease in dendritic intersections in stress + saline treated rats at most of the concentric circles when compared to normal control and stress + OSE treated rats. Similarly, there was a significant (P < 0.05) increase in dendritic intersections at 20 μ concentric circle in stress+4 OSE group when compared to NC group (Table 4B). Dendritic branching points were significantly (P < 0.05 & P < 0.001) decreased in stress + saline treated rats at 2 circles compared to normal control rats. Significant (P < 0.05 & P < 0.001) decrease in branching points was observed in stress + saline treated rats at inner 2 zones when compared to stress+2 ml OSE treated rats. Significant (P < 0.05, P < 0.01 & P < 0.001) decrease in dendritic branching points was also observed in stress + saline treated rats at all zones except 60-80μ compared to stress+4 ml OSE treated rats. However, there was no significant difference in dendritic branching points between NC and stress + OSE treated groups in any of the concentric zones. There was a significant (P < 0.001 & P < 0.0001) reduction in total number of dendritic branching points in stress + saline treated rats compared to normal control and stress + OSE treated rats. However, there was no significant difference in total number of dendritic branching points between NC and stress + OSE treated groups (Table 4C). Dendritic processes arising from cell bodies were significantly (P < 0.01) reduced in saline treated stressed rats compared to stress+4 ml OSE treated rats. However, there was no significant difference in total number of dendritic processes between NC and stress + OSE treated groups (Table 4B).
Table 4B.
Effect of 6 weeks of Ocimum sanctum treatment on 6 week-stressed rats- Substantia nigral neurons– dendritic intersections and processes.
| Groups | n | Distance from soma (μ) |
No. of dendritic processes | ||||
|---|---|---|---|---|---|---|---|
| 20 | 40 | 60 | 80 | 100 | |||
| Normal Control (NC) | 6 | 3.45 ± 0.3 |
5.56 ± 0.54 |
5.83 ± 0.71 |
5.41 ± 0.79 |
4.68 ± 0.4 |
3.05 ± 0.22 |
| Stress + Saline (S→S) | 6 | 3.11 ± 0.33 |
4.43 ∗∗ ± 0.23 |
4.66 ∗∗ ± 0.19 |
4.33 ∗ ± 0.54 |
3.68 ∗ ± 0.75 |
2.83 ± 0.2 |
| Stress+2 ml OSE (S+2-OSE) | 6 | 3.75 a ± 0.28 |
5.51 aa ± 0.36 |
6.21 aaa ± 0.29 |
5.28 a ± 0.6 |
4.5 ± 0.78 |
3.06 ± 0.19 |
| Stress+4 ml OSE (S+4-OSE) | 6 | 4.0 # bb ± 0.65 |
5.95 bbb ± 0.64 |
6.53 bbb ± 0.57 |
5.76 bb ± 0.83 |
4.78 b ± 0.76 |
3.26 bb ± 0.26 |
| F value | 5.06 | 11.54 | 16.86 | 4.56 | 3.14 | 3.85 | |
| P value | P < 0.01 | P < 0.001 | P < 0.0001 | P < 0.05 | P < 0.05 | P < 0.05 | |
Each value represents Mean ± SD; n = number of rats, NC vs. S + S: ∗P < 0.05, ∗∗P < 0.01; NC vs. S+4-OSE: #P < 0.05; S + S vs. S+2-OSE: a P < 0.05, aa P < 0.01, aaa P < 0.001; S + S vs. S+4-OSE: b P < 0.05, bb P < 0.01, bbb P < 0.001; (One way ANOVA, Bonferroni's test).
Table 4C.
Effect of 6 weeks of Ocimum sanctum treatment on 6 week-stressed rats- Substantia nigral neurons – dendritic branching points.
| Groups | n | Concentric zones |
Total dendritic branching points | ||||
|---|---|---|---|---|---|---|---|
| 0-20 μ | 20-40 μ | 40-60 μ | 60-80 μ | 80-100 μ | |||
| Normal Control (NC) | 6 | 0.51 ± 0.18 |
2.35 ± 0.37 |
1.46 ± 0.25 |
0.86 ± 0.28 |
0.55 ± 0.12 |
5.75 ± 0.76 |
| Stress + Saline (S + S) | 6 | 0.38 ± 0.075 |
1.35 ∗∗∗ ± 0.25 |
1.2 ± 0.24 |
0.56 ± 0.18 |
0.23 ∗ ± 0.15 |
3.73 ∗∗∗ ± 0.45 |
| Stress+2 ml OSE (S+2-OSE) | 6 | 0.56 ± 0.12 |
2.46 aaa ± 0.38 |
1.86 a ± 0.41 |
0.78 ± 0.13 |
0.5 ± 0.3 |
6.16 aaaa ± 0.74 |
| Stress+4 ml OSE (S+4-OSE) | 6 | 0.71 b ± 0.3 |
2.53 bbb ± 0.28 |
1.83 b ± 0.59 |
0.76 ± 0.46 |
0.65 bb ± 0.26 |
6.48 bbbb ± 0.58 |
| F value | 3.13 | 17.4 | 3.76 | 1.15 | 3.97 | 22.05 | |
| P value | P < 0.05 | P < 0.0001 | P < 0.05 | NS | NS | P < 0.0001 | |
Each value represents Mean ± SD; n = number of rats, NC vs. S + S: ∗P < 0.05, ∗∗∗P < 0.001; S + S vs. S+2-OSE: a P < 0.05, aaa P < 0.001, aaaa P < 0.0001; S + S vs. S+4-OSE: b P < 0.05, bb P < 0.01, bbb P < 0.001, bbbb P < 0.0001; (One way ANOVA, Bonferroni's test).
4. Discussion
In the present study, the effect of foot shock stress and antistress effect of OSE on rats’ behavior and neurons of substantia nigra were studied. In addition, presence/absence of gastric ulcers (to confirm the stress induction) and body weight gain was also recorded. Fresh extract of O. sanctum leaves was used unlike in other studies [13,14,16] to prevent any structural changes in chemical composition of plant during extraction procedure. Moreover, in traditional/folk medicine, generally crude herbs are used [21]. Further, there are reports of ethanol alone affecting brain regions like substantia nigra [22]. As there are no earlier reports on fresh OSE treatment, 2 doses (2 ml and 4 ml) of OSE were used in this study based on results of our preliminary experiments.
4.1. Attenuation of stress induced gastric ulcers by OSE treatment
Presence of gastric ulcers in all 3 experiments indicated induction of stress on these rats and is in agreement with earlier reports [23,24]. Cellular damage in substantia nigra due to stress may also be a cause of stress-induced ulceration. Bilateral lesions of substantia nigra aggravate stress induced ulcer formation in rats [25]. In the present study, stressed rats treated with OSE were protected from ulcer formation. Anti-ulcerogenic activity of OSE may be due to its ability to reduce acid and increase the mucous secretions and may also be due to its lipoxygenase inhibitory, histamine antagonistic and anti-secretory effects [26,27].
4.2. Effect of stress and OSE treatment on body weight
There was a reduction in the body weight of stressed and stress undergoing rats of all 3 experiment groups. OSE treated stressed and stress-undergoing rats did not show any decrease in the body weight. Similar results showing body weight loss due to stress have been reported by Santos et al. [28]. Retarded body growth may be due to decreased food intake and involvement of corticotrophin releasing factor in hypothalamic region [29]. Decreased body weight loss in OSE treatment groups during and after stress in our study may be due to general health improving properties of OSE [30].
4.3. Effect of stress and OSE treatment on open field test behavior
Foot shock stress for 3 and 6 weeks resulted in the impairments in the locomotory behavior of animals in the open field test. They showed high ambulation, increased amount of time spent in the peripheral zone and frequent entry into central squares indicating stress related anxiety. Such changes in locomotion can be indicative of abnormal brain functioning which could be the result of altered neurological processes in the region like substantia nigra [31]. High ambulatory behavior seen in stressed and untreated rats is an index of low emotionality [19]. Further, stressed rats treated with OSE showed significant improvement in their behavior compared to stressed rats treated with saline. Behavioral scores of OSE treated rats were at par with that of normal control rats indicating attenuation of stress induced behavioral deficits in these rats. This may be by the activity of active principle eugenol present in OSE, which was shown to reduce stress-induced behavioral impairments in rats [16].
4.4. Effect of stress and OSE treatment on dendritic arborization of substantia nigral neurons
Foot shock stress for 3 and 6 weeks decreased dendritic length, branching points and processes of substantia nigral neurons, which is likely to affect the various functions subserved by them. It may result in altered electrical properties, accumulation of 5-HT and dopamine in extracellular region, decreased auto-inhibition and altered excitatory/inhibitory response to afferent signals that may lead to functional deficits after attaining the threshold level. Many mechanisms are involved in such stress induced neurodegenerative changes. Glucocorticoid induced dendritic atrophy can be blocked by treatment with an adrenal steroid synthesis blocker, cyanoketone indicating a role for endogenous glucocorticoids in stress-induced dendritic atrophy [32]. Stress associated neuronal damage may be a result of oxidative damage/heat shock factor expression. Stress in male rats causes oxidative damage to lipid, protein and DNA in cerebral cortex, cerebellum, hippocampus and midbrain [33]. Heat shock protein; Hsp 70i plays an important role in enhancing the survival of neurons following stress [34]. Reduction/alterations in expression of Hsp 70i in substantia nigra may be responsible for neuronal damage following stress. Zinc has been implicated in the etiology of certain neurodegenerative disorders such as amyotrophic lateral sclerosis-parkinsonism dementia and Pick's disease [35,36]. The neuronal damage observed in our study may be due to increased accumulation of zinc in substantia nigral neurons. Such an accumulation of zinc has been reported in Parkinson's disease [37]. It is reported that Zn++ can act as a neuroactive substance in substantia nigra and increase the excitability of these neurons [38]. In a stress situation, Zn++ may be accumulated initially as an antioxidant and on reaching toxic levels, it may act as a neurotoxic substance. Zinc may be released from the axon terminals in the substantia nigra during stress. It is released normally from nerve terminals following electrical stimulation or neuronal activity [39,40]. Results of present study showed a remarkable protection by OSE to the substantia nigral neurons in stressed rats. This activity of OSE may be a direct nullifying action on the probable mechanisms of stress induced neuronal damage discussed earlier, especially glucocorticoid toxicity and excitotoxicity.
It has been reported that pretreatment with O. sanctum prevents stress induced increase in serotonin levels of brain [15]. Neuroprotective activity of OSE may be because of its ability to prevent stress-induced elevations in levels of serotonin. Similarly, OSE is known to prevent stress-induced decrease in levels of excitatory neurotransmitters, adrenaline and noradrenaline [15]. High turnover of noradrenaline during stress is blocked by ethanol in substantia nigra [22]. It is possible that OSE may also act like ethanol in reducing the excitatory activity. Normalizing action of plant extract on level of adrenaline and noradrenaline may be helping the body to cope up better during stress.
Stress is known to stimulate zinc release in brain which exerts its neurotoxic effects by potentiating non-NMDA receptor mediated excitotoxic injury [41]. Neuroprotective activity of ascorbic acid against zinc-induced neurotoxicity is reported in cultured retinal neurons [42]. O. sanctum prevents stress-induced decrease in levels of ascorbic acid content in mice [13]. By preventing stress-induced decrease in ascorbic acid level, O. sanctum may protect substantia nigral neurons against glutamate and zinc toxicity. Jothie et al. have established that the anti-stress activity of OSE could be because of inhibition of release of cortisol, antagonizing the activity of CRHR1 receptor and also by inhibiting 11β-hydroxysteroid dehydrogenase type 1 and Catechol-O-methyltransferase activities [43]. The n-butanol-soluble fractions derived from O. sanctum namely Ocimumoside A and Ocimumoside B are reported to have anti-stress property which could be due to their corticosterone like effect [44].
Oxidative stress is one of the causes of neuronal damage [36]. Antioxidant role of components of O. sanctum like Cirsilineol, isothymusin, isothymonin and rosmarinic acid extract is well known [45]. Anti-oxidant property of two flavonoids of O. sanctum; orientin and vicenin which are known for free radical scavenging activity could be involved in protection of substantia nigral neurons.
O. sanctum prevents stress-induced decrease in Succinate dehydrogenase (SDH) level in liver which is believed to be an indicator of energy metabolism and it increases during stressful situations [14]. This suggests that O. sanctum facilitates conservation of energy in cellular system that could help in adaptive process during stress. Increased levels of SDH in brain may have neuroprotective role, which is enhanced by the anti-stress drugs. O. sanctum may protect the neurons by its cytoprotective activity i.e. by increasing cellular metabolism in neurons thereby making them more stable to face stress-induced insults. Hence, O. sanctum has been included in the list of medicinal plants with anti-stress agents [46].
This study confirms not only the anti-stress role of OSE but also its effect on dendrites of substantia nigral neurons in rats. Prevention and attenuation of deleterious effect of stress on substantia nigra by this plant extract justifies its use in treatment of stress and stress related neurological disorders. Further, correlation between anti-stress activity and effect on dendritic arborization is likely to throw more light on the mechanism of action of O. sanctum. Moreover, the effect of this plant extract on dendritic arborization observed in this study can be a good parameter to study other anti-stress drugs.
5. Conclusions
Both 3 and 6 weeks of foot shock induced neuronal damage in the substantia nigra of rats. Treatment with fresh leaf extract of O. sanctum could prevent and attenuate the foot shock stress induced behavioral deficit and substantia nigral neuronal damage.
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Levine S., Madden J., Conner R.L., Moskal J.R., Anderson D.C. Physiological and behavioral effects of prior aversive stimulation (preshock) in the rat. Physiol Behav. 1973;10:467–471. doi: 10.1016/0031-9384(73)90207-2. [DOI] [PubMed] [Google Scholar]
- 2.Steenbergen H.L., Farabollini F., Heinsbroek R.P.W., Van de Poll N.E. Sex-dependent effects of aversive stimulation on holeboard and elevated plus-maze behavior. Behav Brain Res. 1991;43(2):159–165. doi: 10.1016/s0166-4328(05)80066-x. [DOI] [PubMed] [Google Scholar]
- 3.Paré W.P., Glavin G.B. Restraint stress in biomedical research: a review. Neurosci Biobehav Rev. 1986 Fall;10(3):339–370. doi: 10.1016/0149-7634(86)90017-5. PMID: 3095718. [DOI] [PubMed] [Google Scholar]
- 4.Danner H., Pfister C. Sieben neurontypenin der Substantia nigra der Ratte. Eine golgi-rapid impragnationsstudie. J fur Hirnforsch. 1982;23:553–556. PMID: 6186738. [PubMed] [Google Scholar]
- 5.Juárez Olguín H., Calderón Guzmán D., Hernández García E., Barragán Mejía G. The role of dopamine and its dysfunction as a consequence of oxidative stress. Oxid Med Cell Longev. 2016;2016 doi: 10.1155/2016/9730467. Epub 2015 Dec 6. PMID: 26770661; PMCID: PMC4684895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dallé E., Mabandla M.V. Early life stress, depression and Parkinson's disease: a new approach. Mol Brain. 2018 Mar 19;11(1):18. doi: 10.1186/s13041-018-0356-9. PMID: 29551090; PMCID: PMC5858138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsuwaki T., Yamanouchi K., Nishihara M. The effect of glucocorticoids on bradykinesia induced by immobilization stress. Horm Behav. 2008 Jun;54(1):41–46. doi: 10.1016/j.yhbeh.2008.01.005. Epub 2008 Feb 7. PMID: 18342862. [DOI] [PubMed] [Google Scholar]
- 8.Wu P.Y., Yang X., Wright D.E., Christianson J.A. Foot shock stress generates persistent widespread hypersensitivity and anhedonic behavior in an anxiety-prone strain of mice. Pain. 2020 Jan;161(1):211–219. doi: 10.1097/j.pain.0000000000001703. PMID: 31568043; PMCID: PMC6923604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karakilic A., Kizildag S., Kandis S., Guvendi G., Koc B., Camsari G.B., et al. The effects of acute foot shock stress on empathy levels in rats. Behav Brain Res. 2018 Sep 3;349:31–36. doi: 10.1016/j.bbr.2018.04.043. Epub 2018 Apr 27. PMID: 29709611. [DOI] [PubMed] [Google Scholar]
- 10.Mohandas Rao K.G., Muddanna Rao S., Gurumadhva Rao S. Centella asiatica (L.) leaf extract treatment during the growth spurt period enhances hippocampal CA3 neuronal dendritic arborization in rats. Evid Based Complement Alternat Med. 2006;3(3):349–357. doi: 10.1093/ecam/nel024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nadkarni K.M. ume 1. Popular Prakashan; India: 1996. p. 2. (Nadkarni's Indian materia medica: with Ayurvedic, Unani-Tibbi, Siddha, allopathic, homeopathic, naturopathic & home remedies, appendices & indexes). [Google Scholar]
- 12.Sharma P.C., Yelne M.B., Dennis T.J. Documentation and Publication Division, Central Council for Research in Ayurveda and Siddha, Jawahar Lal Nehru Bharatiya Chikitsa Avum; New Delhi, India: 2005. Database on medicinal plants used in Ayurveda; pp. 500–504. [Google Scholar]
- 13.Bhargava K.P., Singh N. Anti-stress activity of Ocimum sanctum Linn. Indian J Med Res. 1981;73:443–451. PMID: 7275241. [PubMed] [Google Scholar]
- 14.Dadkar V.N., Joshi A.G., Juguste V.S., Billimoria F.R., Dhar H.L. Antistress activity of Ocimum sanctum (tulsi) Indian Drugs. 1987;25(5):172–175. [Google Scholar]
- 15.Singh N., Misra N., Srivastava A.K., Dixit K.S., Gupta G.P. Effect of antistress plants on biochemical changes during stress reaction. Indian J Pharmacol. 1991;23:137–142. [Google Scholar]
- 16.Sen P., Maiti P.C., Puri S., Ray A. Mechanism of antistress activity of Ocimum sanctum Linn. Eugenol and Tinospora malabarica in experimental animals. Indian J Exp Biol. 1992;30(7):592–596. PMID: 1459632. [PubMed] [Google Scholar]
- 17.Mandal S., De K., Chaudhuri S.B., Chowdhury M.K., Sahana C.C. Chemical and pharmacological studies of Ocimum sanctum Linn. on stress adaptation. Indian J Pharmacol. 1990;22:57–58. [Google Scholar]
- 18.Gadahad M.R., Rao M., Rao G. Enhancement of hippocampal CA3 neuronal dendritic arborization by Centella asiatica (Linn) fresh leaf extract treatment in adult rats. J Chin Med Assoc. 2008;71(1):6–13. doi: 10.1016/s1726-4901(08)70066-2. PMID: 18218554. [DOI] [PubMed] [Google Scholar]
- 19.Seibenhener M.L., Wooten M.C. Use of the open field maze to measure locomotor and anxiety-like behavior in mice. JoVE. 2015 doi: 10.3791/52434. PMID: 25742564; PMCID: PMC4354627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohandas Rao K.G., Rao M.S., Rao G.S. Evaluation of amygdaloid neuronal dendritic arborization enhancing effect of Centella asiatica (Linn) fresh leaf extract in adult rats. Chin J Integr Med. 2012 doi: 10.1007/s11655-012-1235-3. PMID. [DOI] [PubMed] [Google Scholar]
- 21.Mohandas Rao K.G., Muddanna Rao S., Gurumadhva Rao S. Enhancement of amygdaloid neuronal dendritic arborization by fresh leaf juice of Centella asiatica (linn) during growth spurt period in rats. Evid Based Complement Alternat. 2009;6(2):203–210. doi: 10.1093/ecam/nem079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shirao I., Tsuda A., Ida Y., Tsujimaru S., Satoh H., Oguchi M., et al. Effect of acute ethanol administration on noradrenaline metabolism in brain regions of stressed and nonstressed rats. Pharmacol Biochem Behav. 1988 Jul;30(3):769–773. doi: 10.1016/0091-3057(88)90097-4. PMID: 3211986. [DOI] [PubMed] [Google Scholar]
- 23.Uno H., Tarara R., Else J.G., Suleman M.A., Sapolsky R.M. Hippocampal damage associated with prolonged and fatal stress in primates. J Neurosci. 1989;9:1705–1711. doi: 10.1523/JNEUROSCI.09-05-01705. 1989. PMID: 2723746; PMCID: PMC6569823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henke P.G. Hippocampal pathway to the amygdala and stress ulcer development. Brain Res Bull. 1990;25(5):691–695. doi: 10.1016/0361-9230(90)90044-z. PMID: 2289157. [DOI] [PubMed] [Google Scholar]
- 25.Ray A., Henke P.G., Sullivan P.M. Central dopamine synthesis and gastric stress pathology in rats. Physiol Behav. 1988;42(4):359–364. doi: 10.1016/0031-9384(88)90277-6. PMID: 3387489. [DOI] [PubMed] [Google Scholar]
- 26.Mandal S., Das D.N., De K., Ray K., Roy G., Chaudhuri S.B., et al. Ocimum sanctum Linn. - a study on gastric ulceration and gastric secretion in rats. Indian J Physiol Pharmacol. 1993;37(1):91–92. PMID: 8449557. [PubMed] [Google Scholar]
- 27.Singh S., Majumdar D.K. Evaluation of the gastric antiulcer activity of fixed oil of Ocimum sanctum (Holy Basil) J Ethnopharmacol. 1999;65(1):13–19. doi: 10.1016/s0378-8741(98)00142-1. PMID: 10350365. [DOI] [PubMed] [Google Scholar]
- 28.Santos J., Bejamin M., Yang P.C., Prior T., Perdue M.H. Chronic stress impairs rat growth and jejunal epithelial barrier function: role of mast cells. Am J Physiol Gastrointest Liver Physiol. 2000;278(6):G847–G854. doi: 10.1152/ajpgi.2000.278.6.G847. PMID: 10859213. [DOI] [PubMed] [Google Scholar]
- 29.Delbende C., Delarue C., Lefebvre H., Bunel D.T., Szafarczyk A., Mocaër E., et al. Glucocorticoids, transmitters and stress. Br J Psychiatr Suppl. 1992 Feb;(15):24–35. PMID: 1356355. [PubMed] [Google Scholar]
- 30.Khosla M.K. Sacred Tulsi (Ocimum sanctum L.) in traditional medicine and pharmacology. Ancient Sci Life. 1995;15(1):53–61. PMID: 22556721; PMCID: PMC3331186. [PMC free article] [PubMed] [Google Scholar]
- 31.Kraeuter A.K., Guest P.C., Sarnyai Z. The open field test for measuring locomotor activity and anxiety-like behavior. Methods Mol Biol. 2019;1916:99–103. doi: 10.1007/978-1-4939-8994-2_9. PMID: 30535687. [DOI] [PubMed] [Google Scholar]
- 32.Magarinos A.M., McEwen B.S. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neurosciences. 1995;69(1):89–98. doi: 10.1016/0306-4522(95)00259-l. PMID: 8637636. [DOI] [PubMed] [Google Scholar]
- 33.Liu J., Wang X., Shigenaga M.K., Yeo H.C., Mori A., Amus B.H. Immobilization stress causes oxidative damage to lipid, protein, and DNA in brain of rats. Faseb J. 1996;10(13):1532–1538. PMID: 8940299. [PubMed] [Google Scholar]
- 34.Uney J.B., Staley K., Tyers P., Sofroniew M.V., Kew J.N. Transfection with hsp70i protects rat dorsal root ganglia neurones and glia from heat stress. Gene Ther. 1994;1(Suppl 1) S65. PMID: 8542411. [PubMed] [Google Scholar]
- 35.Duncan M.W., Marini A.M., Watters R., Kopin I.J., Markey S.P. Zinc, a neurotoxin to cultured neurons, contaminates cycad flour prepared by traditional Guamanian methods. J Neurosci. 1992;12:1523–1537. doi: 10.1523/JNEUROSCI.12-04-01523. 1992. PMID: 1556606; PMCID: PMC6575812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Constantinidis C., Tissot R. In: Glutamate as a neurotransmitter. DiChiara G., Gessa G.L., editors. Raven Press; New York: 1981. Role of glutamate and zinc in the hippocampal lesions of Pick's disease; pp. 413–422. [PubMed] [Google Scholar]
- 37.Dexter D.T., Wells F.R., Lees A.J., Agid F., Agid Y., Jenner P., et al. Increased nigral iron content and alterations in other metal ions occurring in brain in Parkinson's disease. J Neurochem. 1989;52(6):1830–1836. doi: 10.1111/j.1471-4159.1989.tb07264.x. PMID: 2723638. [DOI] [PubMed] [Google Scholar]
- 38.Chung J.M., Chang S.Y., Kim Y.I., Chul Shin H. Zinc increases the excitability of dopaminergic neurons in rat substantia nigra. Neurosci Lett. 2000;286:183–186. doi: 10.1016/s0304-3940(00)01120-4. PMID: 10832015. [DOI] [PubMed] [Google Scholar]
- 39.Assaf S.Y., Chung S.H. Release of endogenous Zn2+ from brain tissue during activity. Nature. 1984;308:734–736. doi: 10.1038/308734a0. PMID: 6717566. [DOI] [PubMed] [Google Scholar]
- 40.Charton G., Rovira C., Ben-Ari Y., Leviel V. Spontaneous and evoked release of endogenous Zn2+ in the hippocampal mossy fiber zone of the rat in situ. Exp Brain Res. 1985;58(1):202–205. doi: 10.1007/BF00238969. PMID: 3987849. [DOI] [PubMed] [Google Scholar]
- 41.Itoh T., Saito T., Fujimura M., Watanabe S., Saito K. Restraint stress-induced changes in endogenous zinc release from the rat hippocampus. Brain Res. 1993;618(2):318–322. doi: 10.1016/0006-8993(93)91283-x. PMID: 8374763. [DOI] [PubMed] [Google Scholar]
- 42.Shankar S.L., Nagaraja B.L., Meti B.L., Raju T.R. Zinc neurotoxicity in primary cultures of retina – attenuation by ascorbic acid. Neurodegeneration. 1994;3:163–168. [Google Scholar]
- 43.Jothie Richard E., Illuri R., Bethapudi B., Anandhakumar S., Bhaskar A., Chinampudur Velusami C., et al. Anti-stress activity of Ocimum sanctum: possible effects on hypothalamic-pituitary-adrenal Axis. Phytother Res. 2016 May;30(5):805–814. doi: 10.1002/ptr.5584. Epub 2016 Feb 22. PMID: 26899341. [DOI] [PubMed] [Google Scholar]
- 44.Ahmad A., Rasheed N., Chand K., Maurya R., Banu N., Palit G. Restraint stress-induced central monoaminergic & oxidative changes in rats & their prevention by novel Ocimum sanctum compounds. Indian J Med Res. 2012 Apr;135(4):548–554. PMID: 22664506; PMCID: PMC3385242. [PMC free article] [PubMed] [Google Scholar]
- 45.Kelm M.A., Nair M.G., Strasburg G.M., DeWitt D.L. Anti-oxidant and cyclooxygenase inhibitory phenolic compounds from Ocimum sanctum Linn. Phytomedicine. 2000;7(1):7–13. doi: 10.1016/S0944-7113(00)80015-X. PMID: 10782484. [DOI] [PubMed] [Google Scholar]
- 46.Vaidya A.D.B. The status and scope of Indian medicinal plants acting on central nervous system. Indian J Pharmacol. 1997;29:340–343. [Google Scholar]