Key Points
Question
Does an entirely remote hypertension and hypercholesterolemia program implemented across a large diverse health network help ensure equal access to care?
Findings
In this cohort study including 10 803 patients with blood pressure and/or cholesterol levels above guideline-recommended targets, remote medication titration management was significantly associated with decreased blood pressure and low-density lipoprotein cholesterol compared with education only. Similar rates of enrollment and reductions in blood pressure and low-density lipoprotein cholesterol were observed across different racial, ethnic, and primary language groups.
Meaning
These results highlight that multiple diverse populations may be treated effectively with digitally enabled remote care programs.
This cohort study evaluates the efficacy of a remote hypertension and cholesterol management program vs education only among patients with hypertension and/or high cholesterol in a diverse health care network.
Abstract
Importance
Blood pressure (BP) and cholesterol control remain challenging. Remote care can deliver more effective care outside of traditional clinician-patient settings but scaling and ensuring access to care among diverse populations remains elusive.
Objective
To implement and evaluate a remote hypertension and cholesterol management program across a diverse health care network.
Design, Setting, and Participants
Between January 2018 and July 2021, 20 454 patients in a large integrated health network were screened; 18 444 were approached, and 10 803 were enrolled in a comprehensive remote hypertension and cholesterol program (3658 patients with hypertension, 8103 patients with cholesterol, and 958 patients with both). A total of 1266 patients requested education only without medication titration. Enrolled patients received education, home BP device integration, and medication titration. Nonlicensed navigators and pharmacists, supported by cardiovascular clinicians, coordinated care using standardized algorithms, task management and automation software, and omnichannel communication. BP and laboratory test results were actively monitored.
Main Outcomes and Measures
Changes in BP and low-density lipoprotein cholesterol (LDL-C).
Results
The mean (SD) age among 10 803 patients was 65 (11.4) years; 6009 participants (56%) were female; 1321 (12%) identified as Black, 1190 (11%) as Hispanic, 7758 (72%) as White, and 1727 (16%) as another or multiple races (including American Indian or Alaska Native, Asian, Native Hawaiian or Other Pacific Islander, unknown, other, and declined to respond; consolidated owing to small numbers); and 142 (11%) reported a preferred language other than English. A total of 424 482 BP readings and 139 263 laboratory reports were collected. In the hypertension program, the mean (SD) office BP prior to enrollment was 150/83 (18/10) mm Hg, and the mean (SD) home BP was 145/83 (20/12) mm Hg. For those engaged in remote medication management, the mean (SD) clinic BP 6 and 12 months after enrollment decreased by 8.7/3.8 (21.4/12.4) and 9.7/5.2 (22.2/12.6) mm Hg, respectively. In the education-only cohort, BP changed by a mean (SD) −1.5/−0.7 (23.0/11.1) and by +0.2/−1.9 (30.3/11.2) mm Hg, respectively (P < .001 for between cohort difference). In the lipids program, patients in remote medication management experienced a reduction in LDL-C by a mean (SD) 35.4 (43.1) and 37.5 (43.9) mg/dL at 6 and 12 months, respectively, while the education-only cohort experienced a mean (SD) reduction in LDL-C of 9.3 (34.3) and 10.2 (35.5) mg/dL at 6 and 12 months, respectively (P < .001). Similar rates of enrollment and reductions in BP and lipids were observed across different racial, ethnic, and primary language groups.
Conclusions and Relevance
The results of this study indicate that a standardized remote BP and cholesterol management program may help optimize guideline-directed therapy at scale, reduce cardiovascular risk, and minimize the need for in-person visits among diverse populations.
Introduction
Elevated blood pressure (BP) and levels of low-density lipoprotein cholesterol (LDL-C) are well known atherosclerotic cardiovascular risk factors.1,2 Their control, through dietary, lifestyle, and pharmacotherapeutic interventions, reduces the incidence of atherosclerotic cardiovascular disease (ASCVD) and mortality. Approximately 30% to 50% of eligible patients in the US do not achieve optimal guideline-directed treatment goals.1,3,4 Recent data suggest that rates of BP and lipid control have stagnated and even deteriorated.5,6,7,8 The rate of risk factor control is even lower among certain racial and ethnic populations, those who live in rural geographic areas, and those with limited English proficiency.9,10,11,12
Multiple system-, clinician-, and patient-level factors prevent effective longitudinal medical management of hypertension and hyperlipidemia, including ineffective patient identification and risk stratification, inadequate access to care, clinical inertia, heterogeneous treatment choices, lack of integrated monitoring devices, and poor data collection and integration. The current health care delivery model exacerbates much of the heterogeneity in care, underrecognizes population risk, and permits persistent disparities in care.11,13,14 Over the past 5 years, our care delivery team has developed a series of remotely delivered chronic disease management programs driven by standardized medical and workflow algorithms, staffed by trained but nonlicensed navigators and licensed pharmacists, and backed by the professional staff of the Mass General Brigham (MGB) health care system.15,16,17 A variety of digital solutions support the clinical workflows to improve efficiency, enforce standardization, expand communication channels, and improve data collection and analysis. Through these efforts, we aim to lower barriers to care and increase access for patients from traditionally underserved populations.
We demonstrated that this model was associated with a reduction in hypertension and hyperlipidemia18; however, whether this model of remote care delivery is scalable at a population level and whether it meets the needs of a diverse patient population has yet to be shown. Age, race, ethnicity, and language create barriers to telehealth that are challenging to overcome.19,20 With the shift toward greater remote patient interaction and care delivery following the COVID-19 pandemic, concerns about further exacerbating existing inequities with telemedicine and digital health have been raised.21,22 In this report, we present results from among the first 10 000 patients enrolled in a remote hypertension and LDL-C management program across a diverse population in the MGB health care network.
Methods
Population
The remote BP and LDL-C management programs are part of an ongoing clinical implementation project within the MGB health care system. A report describing our program’s characteristics and outcomes has been published previously.18 We provide a comprehensive management solution, including disease identification, patient engagement, device integration, education, laboratory assessment, and medication initiation and titration. Nonlicensed navigators supervised by a team of pharmacists, nurse practitioners, and physicians coordinate care using customer relationship management software, streamlined task automation, clinical decision support algorithms, and omnichannel communication.23 Home BP measurements and laboratory values are monitored daily for disease management and safety. This study was approved by the institutional review board at Mass General Brigham as a quality improvement program, with approval for data collection and analyses. Patients provided verbal consent for clinical participation in the program but the need for written consent was waived as this was considered a quality improvement program delivering standard of care.
Eligibility and Enrollment
Patients were eligible if they were monitored by a physician within the MGB system (1 visit or more within the prior 3 calendar years), aged 26 to 80 years, and had BP and/or LDL-C above guideline-recommended targets.1,2 Patients enter the program through direct referral via electronic health record (EHR) through population screening. Physicians of potentially eligible patients are contacted to solicit appropriateness and can decline inclusion for any patients they do not feel are good candidates for the program.
Patients eligible for hypertension evaluation and management must have at least 2 BP readings 130 mm Hg systolic BP (SBP) or greater and/or 80 mm Hg diastolic BP (DBP) or greater in the EHR within the last 18 months not related to emergency department, urgent care, or procedural visits. For referrals, we accept patients with BP readings 130 mm Hg SBP or greater and/or 80 mm Hg DBP or greater in the EHR within the last 18 months not related to emergency, urgent care visit, or procedural visits or an ambulatory 24-hour BP mean of 130/80 mm Hg or greater. The mean of the highest 2 of the most recent 3 BP measures taken with the above exclusion criteria is deemed the program-qualifying BP.
Patients met criteria for cholesterol lowering based on 4 class I/level A criteria for recommended LDL-C therapy: age 26 to 80 years with LDL-C 70 mg/dL (to convert to mmol/L, multiply by 0.0259) or greater and clinical ASCVD; age 26 to 80 years with severe hypercholesterolemia (any historical LDL-C 190 mg/dL or greater); age 40 to 75 years with diabetes or hemoglobin A1c greater than 6.5% and LDL-C 70 to 189 mg/dL; age 40 to 75 years with LDL-C 100 to 189 mg/dL and a calculated 10-year ASCVD risk of 7.5% or higher.2,24 Further details on the characteristics of this program have been published previously.16,17,18
Patient Engagement and Treatment
Qualified patients were contacted by navigators to confirm patient eligibility and interest in participation. The patient navigator plays the primary role of communicating, collecting data, and conveying dietary, lifestyle, and medication recommendations made by the clinical team designed to optimize risk reduction in patients. Additional details regarding the patient navigators are in the eMethods in the Supplement.
Patients enrolled in the hypertension program received a BP cuff that was either Bluetooth enabled to pair with a smartphone app or cellular enabled to automatically send BP measurements. After education on proper BP ascertainment technique, a program baseline BP was established, during which patients were asked to obtain 2 morning and 2 evening BP readings for 6 days. If the mean program baseline BP was above the patient’s BP goal, the patient was provided with dietary, lifestyle, education, and medication recommendations as well as a prescription. Baseline laboratory data were obtained if there were none within the prior 12 months. There were more than 424 000 BP readings recorded as part of the hypertension program, with patients sending a mean (SD) of 0 (17.0) readings per month. The median (IQR) time in the hypertension program for all participants was 103 (50-192) days. Completing the program was termed reaching the maintenance phase, defined as cessation of active titration due to achievement or close approximation (within 1 to 2 mm Hg) of goal BP or (5 to 10 mg/dL) LDL-C. Transitioning a patient who was not quite at goal BP or LDL-C to maintenance resulted from clinical discussions based on medical judgment and patient preference.
Patients enrolled in the lipids program were predominately identified by EHR population-level screening. Program baseline LDL-C was the most recent LDL-C within prior 18 months, or if no measures were present at enrollment, a new sample was obtained. Navigators then followed the drug-treatment algorithm to initiate and titrate therapy, monitor laboratory values and symptoms for the safety and tolerability of therapy, and achieve LDL-C targets. The median time in the lipids program for all participants was 131 (70-234) days.
Medication initiations and titrations were based on an established drug-treatment algorithm as part of collaborative drug therapy management agreements with statutes allowing pharmacists to prescribe under a supervising physician with the use of disease-specific protocols25 (eFigures 1 and 2 in the Supplement). Scenarios outside the prescribed medication algorithm were routed to the supervising physician, and changes were signed off by a pharmacist and communicated to the patient and care team by a patient navigator under the supervision of nurse practitioners or physicians. After each change in medication, reassessment of BP and laboratory monitoring were collected in an iterative process until targets were achieved.1
Cohort Designation
The education-only cohort represented a group of 1266 patients who agreed to receive dietary, lifestyle, and medication advice, but declined to participate in home BP monitoring and program medication titration. This population became a nonrandomized concurrent control cohort in which we compared BP and LDL-C with the enrolled cohort who initially agreed to medication management. Because the education-only cohort did not have any home BP readings, only office BP readings extracted from the EHR nearest the appropriate time points were used for this comparison for both groups.
Data
Clinical data were extracted from the MGB enterprise data warehouse and confirmed by medical record review. This analysis included all patients enrolled before July 1, 2021, with data locked as of December 1, 2021. Data analysis for this project was approved by the MGB Institutional Review Board.
Statistical Analysis
Categorical variables are reported as frequencies and proportions and were compared using χ2 or Fisher exact tests, as appropriate. Continuous variables are reported as means with standard deviations or medians as appropriate and were compared using 2-tailed t tests or Mann-Whitney U tests, as appropriate. A P value less than .05 was considered significant.
Results
Patient Disposition and Demographic Characteristics
Between January 1, 2018, and July 1, 2021, we screened 20 454 patients, contacted 18 444, and enrolled 10 803 in our remote hypertension and lipids medication management programs (eFigure 3 in the Supplement). For the hypertension program, 182 857 patients were identified as potentially eligible based on EHR data including BPs and clinical history; 5046 were contacted and 3658 enrolled. Of patients who enrolled, 2414 took requisite baseline BP measurements (minimum 12 in 1 week) to establish a program baseline and continue in the program. In the hypertension program, the mean (SD) office BP prior to enrollment was 150/83 (18/10) mm Hg, and the mean (SD) home BP was 145/83 (20/12) mm Hg. After program baseline measurements, 651 were found to have home BP at goal and therefore required no further medication titration. At the time of data lock, 524 patients remained active in the program, 1064 became unreachable, 302 returned to the care of their primary care physician for adherence issues or challenges in communication, 276 dropped out, and 1492 achieved their goal and entered maintenance.
In the lipid program, 240 596 patients were identified as eligible by EHR databased on LDL-C levels and clinical history, 27 118 were screened, 15 583 were contacted, and 8103 were enrolled. All program participants had a cholesterol value obtained within the prior 12 months of enrollment. At the time of data lock, 1572 patients remained active in the program, 2528 became unreachable, 817 dropped out of remote management, 433 returned to the care of their primary care physician for adherence issues or challenges in communication, and 2753 achieved their goal and entered maintenance.
Baseline Demographic Characteristics
The mean (SD) age of the enrolled population was 65 (11.4) years; 6009 participants (56%) were female; 1321 (12%) identified as Black, 1190 (11%) as Hispanic, 7758 (72%) as White, and 1727 (16%) as another or multiple races (including American Indian or Alaska Native, Asian, Native Hawaiian or Other Pacific Islander, unknown or other race, and those who declined to respond; consolidated because numbers were too small to power subgroup analyses); and 142 (11%) reported a preferred language other than English (eTable 1 in the Supplement). Baseline demographic characteristics of patients in the education-only and medication management cohorts are presented in eTable 4 in the Supplement. Patients in the medication management cohort were older, with a higher proportion of Black and Hispanic participants and participants with a reported preferred language other than English compared with those in the education-only cohort.
Operational Data
For the hypertension program, we received 424 482 BP values and 59 867 laboratory results and issued 15 047 new prescriptions for a mean of 2.5 medication changes per patient. In the cholesterol program, we reviewed 79 396 laboratory results and issued 12 838 new prescriptions—a mean of 1.7 medication changes per patient. The number of activities required to reach maintenance per patient are presented in eTable 2 in the Supplement.
Change in Office BP and LDL-C in the Education-Only Cohort Compared With the Medication Management Cohort
The mean (SD) initial BP (BP taken in the office closest to first contact with the program) was 140.4/78.7 (16.7/9.7) mm Hg in the education-only cohort and 144.4/81.9 (17.1/11.3) mm Hg in the medication management cohort. Compared with patients in the education-only cohort, mean (SD) office SBP and DBP were significantly lower in the medication management cohort at 6 months (−1.5/−0.7 [23.0/11.1] mm Hg vs. −8.7/−3.8 [21.4/12.4] mm Hg; P < .001) and 1 year (+0.2/−1.9 [30.3/11.2] mm Hg vs −9.7/−5.2 [22.2/12.6] mm Hg; P < .001) (Table 1). Similarly, the mean (SD) LDL-C reduction was less in the education-only cohort compared to the medication management cohort both at 6 and 12 months (−9.3 [34.3] vs −35.4 [43.1] mg/dL) and at 1 year (−10.2 [35.5] vs −37.5 [43.9] mg/dL) (P < .001) (Table 1).
Table 1. Blood Pressure (BP) and Lipid Outcomes Among Patients Enrolled in the Hypertension and Cholesterol Program by Education Only and Medication Management Cohorts.
| Outcome | Education only | No. | Change from initial value | Medication management | No. | Change from initial value | P value for between-group change |
|---|---|---|---|---|---|---|---|
| Office systolic BP outcomes, mean (SD), mm Hg | |||||||
| Initial BP | 140.4 (16.7) | 301 | NA | 144.4 (17.1) | 3370 | NA | NA |
| 6-mo BP | 139.9 (18.6) | 284 | −1.5 (23.0) | 135.7 (17.4) | 3284 | −8.7 (21.4) | <.001 |
| 1-y BP | 140.6 (27.5) | 263 | 0.2 (30.3) | 134.7 (17.6) | 2952 | −9.7 (22.2) | <.001 |
| Office diastolic BP outcomes, mean (SD), mm Hg | |||||||
| Initial BP | 78.7 (9.7) | 301 | NA | 81.9 (11.3) | 3370 | NA | NA |
| 6-mo BP | 78.0 (11.3) | 284 | −0.7 (11.1) | 78.1 (10.6) | 3284 | −3.8 (12.4) | <.001 |
| 1-y BP | 76.8 (11.1) | 263 | −1.9 (11.2) | 76.7 (10.6) | 2952 | −5.2 (12.6) | <.001 |
| Lipid outcomes, mean (SD), mg/dL | |||||||
| Baseline LDL | 131.3 (42.0) | 965 | NA | 144.0 (47.0) | 7138 | NA | NA |
| 6-mo LDL | 122.0 (47.0) | 965 | −9.3 (34.3) | 108.6 (44.8) | 6347 | −35.4 (43.1) | <.001 |
| 1-y LDL | 121.1 (46.1) | 887 | −10.2 (35.5) | 106.5 (45.1) | 5464 | −37.5 (43.9) | <.001 |
Abbreviations: LDL, low-density lipoprotein; NA, not applicable.
Change in Blood Pressure and LDL-C After Enrollment in the Medication Management Cohort
Among all enrolled patients (excluding those in the education-only cohort) who agreed to receive medication management, mean (SD) home BP at program exit was significantly lower than qualifying BP (SBP −15.6 [20.7] mm Hg and DBP−5.8 [11.7] mm Hg; P < .001). Mean (SD) exit BP was also significantly lower than baseline program BP (SBP −9.9 [14.2] mm Hg and DBP −6.0 [8.2] mm Hg; P < .001). Absolute BP reductions were greater in patients who successfully entered maintenance and in patients found to have hypertension on home baseline BP readings (Table 2). There were 117 patients with hypertensive office BPs but home BP readings at goal and 534 patients who were hypertensive and taking pharmacotherapy with home BP readings at goal but with hypertensive office BP readings. For patients who exited prematurely, became unreachable, or were still active in the program after 5 months, the last received mean (SD) home BP was significantly lower than the program baseline, with reductions in SBP of −7.1 (13.3) mm Hg and DBP of −3.8 (7.4) mm Hg BP. For patients who reached maintenance, the median (IQR) time in the program was 86 (38-174) days. For patients who exited without reaching maintenance, the median (IQR) time in the program was 116 (65-209) days. eFigure 4A in the Supplement presents the number of hypertension agents prescribed from baseline to program completion. Greater BP control was achieved with higher doses and a greater number of BP agents.
Table 2. Systolic Blood Pressure (SBP) and Diastolic Blood Pressure (DBP) Outcomes Among Patients Enrolled in the Hypertension Program by Program Milestone Achieved.
| BP, mean (SD), mm Hga | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Qualifying | Program baseline | Final program | Qualifying to final program | Baseline to final program | ||||||
| SBP | DBP | SBP | DBP | SBP | DBP | SBP reduction | DBP reduction | SBP reduction | DBP reduction | |
| All enrolled patients (N = 3658) | ||||||||||
| All patients | 150.3 (17.6) | 83.3 (10.0) | 145.4 (20.0) | 83.2 (12.0) | 135.2 (17.7) | 77.3 (10.4) | 14.9 (20.5) | 5.6 (11.7) | 10.2 (14.3) | 5.7 (8.4) |
| Sustained hypertension with hypertensive program baseline BP | 150.8 (18.9) | 83.4 (10.2) | 151.6 (17.6) | 86.5 (11.0) | 138.6 (18.1) | 79.1 (10.5) | 12.2 (20.9) | 4.1 (11.9) | 13.1 (14.6) | 7.2 (8.6) |
| Exited prematurely or became unreachable (n = 1697) | ||||||||||
| All patients | 154.3 (18.7) | 85.0 (10.7) | 155.6 (19.4) | 87.8 (11.1) | 148.3 (18.2) | 83.8 (10.7) | 6.0 (22.1) | 1.1 (12.4) | 7.4 (13.2) | 4.0 (7.4) |
| Sustained hypertension with hypertensive program baseline BP | 156.1 (20.0) | 84.8 (10.8) | 156.5 (18.9) | 88.4 (10.7) | 149.0 (18.2) | 84.4 (10.4) | 7.0 (22.7) | 0.4 (12.4) | 7.1 (13.3) | 4.0 (7.3) |
| Still active (n = 444) | ||||||||||
| All patients | 150.2 (13.1) | 84.1 (8.3) | 148.5 (15.1) | 89.7 (7.9) | NA | NA | NA | NA | NA | NA |
| Sustained hypertension with hypertensive program baseline BP | 150.5 (13.4) | 87.1 (7.0) | 148.9 (14.8) | 89.9 (7.7) | NA | NA | NA | NA | NA | NA |
| Entered maintenance (n = 1517) | ||||||||||
| All patients | 146.0 (15.8) | 81.4 (9.1) | 137.5 (17.0) | 79.3 (11.5) | 124.8 (7.5) | 72.2 (6.5) | 21.1 (16.8) | 9.2 (9.6) | 12.6 (14.5) | 7.1 (8.8) |
| Sustained hypertension with hypertensive program baseline BP | 144.8 (16.1) | 81.4 (9.4) | 146.3 (14.4) | 84.0 (11.1) | 126.6 (7.2) | 73.1 (6.7) | 18.2 (16.7) | 8.3 (9.7) | 19.7 (13.3) | 10.9 (8.6) |
Abbreviations: BP, blood pressure NA, not applicable.
Qualifying BP measurements were those taken in the office; program baseline and final program BP measurements were taken at home. Patients with hypertensive BP at baseline formed a cohort excluding patients with office readings higher than at-home readings whose baseline home BP reading was at goal.
The mean (SD) observed LDL-C reduction in all enrolled patients was 46.2 mg/dL (53.6) compared to baseline (144.0 [47.0] at baseline vs 98.3 [42.2] at exit; P < .001). Among patients who reached maintenance, the observed mean (SD) LDL-C reduction was from 140 (43.7) mg/dL to 70 (22.3) mg/dL (a 50% reduction), with 2611 patients (94%) achieving their LDL-C goals. In patients who exited prematurely, became unreachable, or remained active in the program after 5 months, a mean (SD) reduction of 25.1 (52.3) mg/dL in LDL-C was achieved. The proportional LDL-C reduction was consistent among the 4 different ASCVD risk categories, but the absolute LDL-C reduction was greatest in patients with any historical LDL-C greater than 190 mg/dL (a mean [SD] reduction of 93 [50.4] mg/dL) (Table 3). The mean (SD) achieved LDL-C in all patients with established ASCVD was 82.6 (37.3) mg/dL in all enrolled patients and 58.4 (17.5) mg/dL in those who reached maintenance. For patients who exited without reaching maintenance, the median (IQR) time in the program was 150 (88-256) days. eFigure 4B in the Supplement presents the lipid-lowering therapy changes from baseline to program completion in patients who reached maintenance. The proportion of patients using high-intensity statin increased from 39.7% (n = 1104) to 54.7% (n = 1520), ezetimibe from 9.2% (n = 256) to 20.0% (n = 556), and PCSK9 inhibitors from 1.1% (n = 31) to 5.4% (n = 151), while among those not receiving any lipid-lowering therapy the proportion decreased from 19.0% (n = 526) to 2.8% (n = 78).
Table 3. Lipid Outcomes Among Patients Enrolled in the Cholesterol Program by Program Milestone Achieved and Clinical Indication for Enrollment.
| LDL, mean (SD), mg/dLa | |||
|---|---|---|---|
| Baseline | Exit | Reduction | |
| All enrolled patients | |||
| Overall | 144.0 (47.0) | 98.3 (42.2) | 46.2 (53.6) |
| ASCVD | 132.8 (46.5) | 81.8 (37.3) | 51.5 (52.7) |
| Diabetes | 133.1 (35.2) | 98.8 (34.8) | 35.3 (45.4) |
| LDL >190 | 181.5 (45.4) | 123.4 (48.7) | 57.4 (63.8) |
| Primary prevention | 136.6 (39.6) | 105.7 (33.1) | 30.8 (43.5) |
| Exited prematurely or became unreachable | |||
| Overall | 148.4 (48.7) | 123.3 (39.4) | 25.1 (52.3) |
| ASCVD | 139.6 (48.6) | 104.9 (36.6) | 34.7 (55.7) |
| Diabetes | 132.3 (33.6) | 119.7 (29.2) | 12.6 (38.1) |
| LDL >190 | 185.5 (47.2) | 155.1 (38.8) | 30.3 (59.8) |
| Primary prevention | 142.5 (47.0) | 128.5 (26.8) | 14.0 (47.7) |
| Still active | |||
| Overall | 133.3 (54.1) | NA | NA |
| ASCVD | 116.8 (50.7) | NA | NA |
| Diabetes | 115.2 (36.9) | NA | NA |
| LDL >190 | 210.2 (34.9) | NA | NA |
| Primary prevention | 137.3 (44.3) | NA | NA |
| Entered maintenance | |||
| Overall | 140.0 (43.7) | 69.4 (22.3) | 70.6 (44.0) |
| ASCVD | 126.8 (42.7) | 57.8 (17.5) | 69.0 (42.9) |
| Diabetes | 136.3 (36.2) | 73.3 (21.4) | 63.0 (37.5) |
| LDL >190 | 174.5 (42.3) | 82.1 (21.9) | 92.4 (50.4) |
| Primary prevention | 130.2 (27.0) | 81.3 (18.8) | 48.9 (29.1) |
Abbreviations: ASCVD, atherosclerotic cardiovascular disease; LDL, low-density lipoprotein.
To convert to mmol/L, multiply by 0.0259.
Program Efficacy Across Diverse Populations
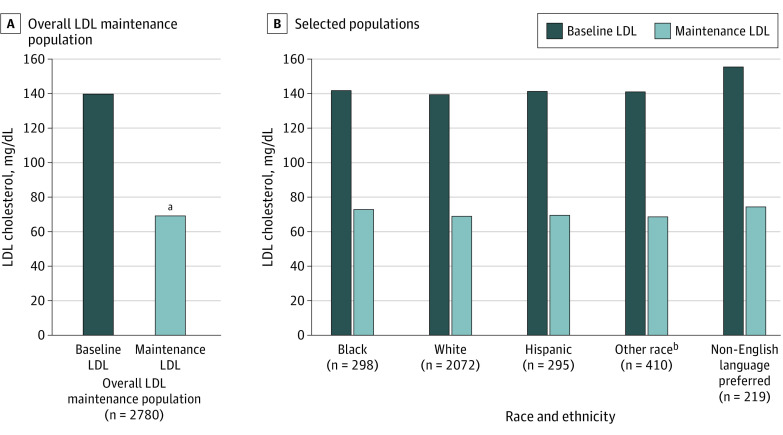
When examining the effect of the hypertension and hyperlipidemia programs on different patient subpopulations, we found similar rates of enrollment and clinical benefits regardless of race, ethnicity, and preferred language (eTable 3 in the Supplement). The mean (SD) BP achieved BP among Black patients (123/73 [9/7] mm Hg), Hispanic patients (125/73 [6/6] mm Hg), and patients with a preferred language other than English (126/73 [6/7] mm Hg) were similar to BP achieved for White patients (126/72 [7/6] mm Hg) (Figure 1). Patients in the lipids program also demonstrated similar LDL-C reductions and LDL-C levels achieved among Black patients (68.6 and 72.9 mg/dL, respectively) Hispanic patients (71.7 and 69.5 mg/dL, respectively) and patients with a preferred language other than English (80.7 and 74.3 mg/dL, respectively) compared with White patients (70.8 and 68.6 mg/dL, respectively) (Figure 2).
Figure 1. Clinical Blood Pressure (BP) Results in Patients Who Reached Maintenance.
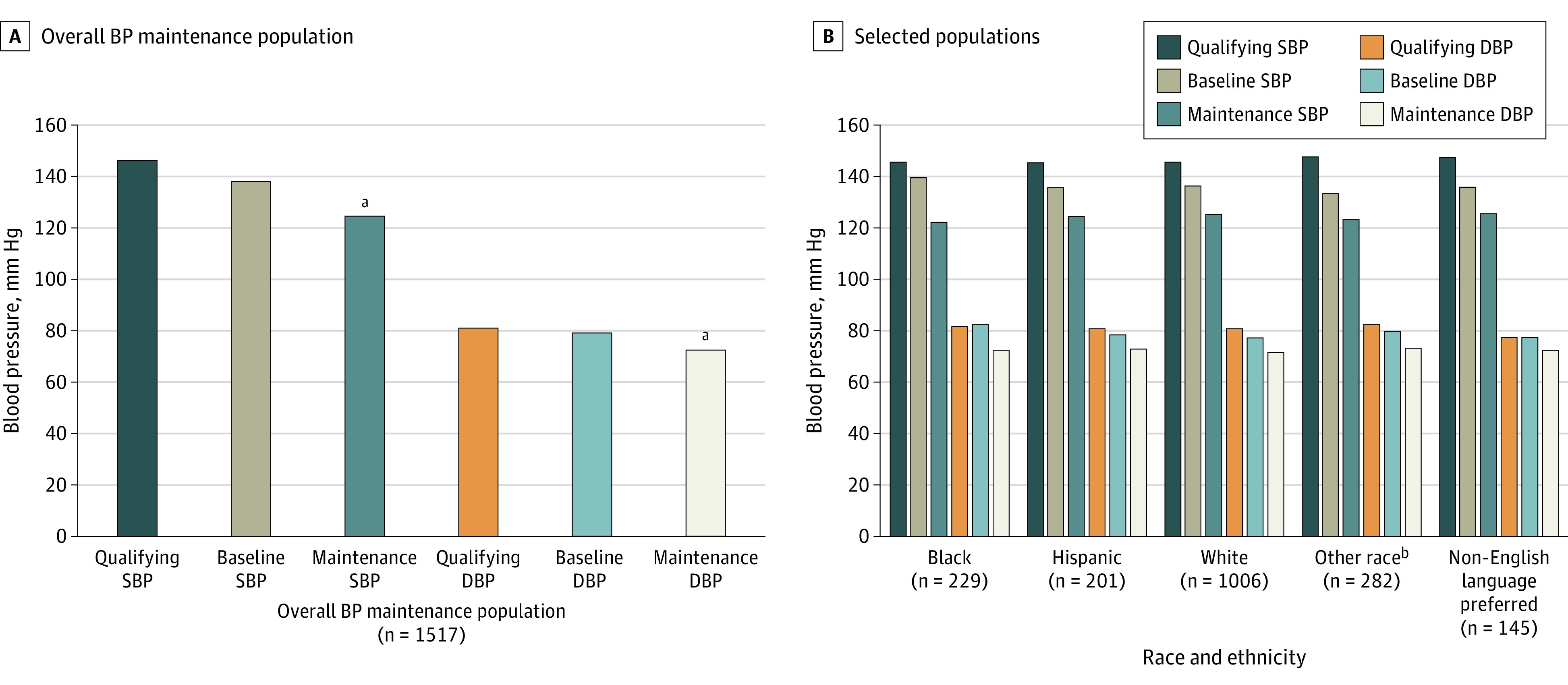
Qualifying BP was defined as the highest 2 of the last 3 office BP readings taken in an outpatient office visit. Baseline BP was the mean of at least 12 BP readings taken over a 1-week period at home. Maintenance BP was defined as the last home BP reading transmitted while still in the program. DBP indicates diastolic blood pressure; SBP, systolic blood pressure.
aP < .001 compared to baseline and qualifying values.
bOther included American Indian or Alaska Native, Asian, Native Hawaiian or Other Pacific Islander, unknown or other race, and those who declined to respond; consolidated because numbers were too small to power subgroup analyses.
Figure 2. Clinical Low-Density Lipoprotein (LDL) Results in Patients Who Reached Maintenance.
LDL reductions in all patients who completed the program (maintenance) and in selected subpopulations.
aP < .001 compared to baseline LDL.
bOther included American Indian or Alaska Native, Asian, Native Hawaiian or Other Pacific Islander, unknown or other race, and those who declined to respond; consolidated because numbers were too small to power subgroup analyses.
Discussion
In this cohort study, we found that enrollment in a remote, navigator-led, pharmacist-supported system-level hypertension and cholesterol optimization program was associated with a reduction in BP and LDL-C in a heterogenous patient population from a large health care network compared with enrollment in an education-only program. Our programs were developed and iteratively modified according to several key objectives, including redesigned treatment paths, remote delivery, frequent patient contact, and more rigorous data collection, curation, and analyses. These activities were supported by technology solutions to increase efficiency, standardization, and productivity.
Telemedicine and remote health delivery services surged during the COVID-19 pandemic due to stay-at-home orders, reduced in-person clinical capacity, and efforts to minimize exposure risk.26,27 With the end of most public health emergency orders and the return to in-patient care, rates of telemedicine services at traditional health care organizations are declining,28,29 even though remote services received surprisingly positive reviews from both patients and health care professionals.30,31,32,33,34Finding sustainable models of remote care delivery to optimize disease management and simultaneously increase access to care remains challenging. Many of the available remote disease management services focus on lifestyle and diet recommendations only (with minimal to no pharmacotherapy), are typically paid through insurance or employers, and require a high degree of digital literacy,35,36 which limits their ultimate therapeutic potential. Self-selection for these programs favors populations with lower rates of chronic disease, morbidity, and mortality, thus further exacerbating disparities in care delivery.37,38,39 Demographic characteristics of patients screened and enrolled in our hypertension and cholesterol programs reflect the overall population within the MGB health system. They also mirror contemporary Massachusetts population characteristics, with 9% of the population identifying as Black, 81% as White, and 10% as another race and 12% identifying as Hispanic.40
Our program focuses on improving care and the patient experience through greater adoption of guideline-supported algorithms, task-shifting, and digital technologies as part of novel clinical workflows. For example, in our hypertension program, while Bluetooth and cellular BP cuffs were offered, most patients preferred cellular-based BP cuffs for ease of setup. The model of pharmacist-driven care is well established through anticoagulation services and in hypertension has demonstrated superior results compared with standard of care.41,42,43,44,45 Standardizing workflows, treatment choices, and data collection enhances the ability to detect deviation, test modifications, and develop a more robust learning health system. Other pharmacy-led approaches targeting underserved populations have demonstrated marked BP improvements. A cluster randomized trial46 of BP reduction in Black barbershops demonstrated a mean reduction of 27.0 mm Hg in SBP (from 152.8 mm Hg to 125 mm Hg) with 84 of 132 participants (63.6%) achieving their goal BP of less than 130/80 mm Hg.
By intention, our program did not require patients to have any technical literacy, illustrated by the fact that phone calls were the predominant method of communication. We used preregistered cellular-enabled blood pressure cuffs, ensuring that no Wi-Fi or smartphone was necessary to transmit readings. We aimed to make this program accessible to all and thus believe that by coupling patients with care navigators to assist when necessary and to remind patients to complete readings, obtain laboratory tests, or start new therapy was likely the reason for the associations observed between program enrollment and clinical outcomes. While we believe that technology allows more efficient use of clinical and nonclinical resources, the combination of workflow automatization, patient-centric design, and personalized patient experience and interaction are integral to our program.
Patient engagement and retention remain a challenge for any remote program.47 While the largest segment of our enrolled patients graduated, a considerable number became unreachable and only completed part of our program. To understand the challenges patients encounter when trying to complete the program, we conducted an internal analysis of 200 randomly sampled patients who became unreachable or dropped out of the program and were able to reach approximately 30% of patients. Many patients reached felt that their condition was under control, preferred to work directly with their physician, were not comfortable with additional medications, or stated the program was not convenient. This analysis highlights areas for improvement in education, health care professional coordination, and patient engagement. Further research on engagement strategies, incentives, and behavioral economics is required to understand reasons for noncompletion and interventions that may be able to promote goal attainment.
Sustainable models to deliver ongoing care will require activating value levers that are different in fee-for-service compared with value-based models. In a value-based care model, improved chronic disease quality metrics result in a reduction of cardiovascular events, leading to overall lower medical expenditures. Conversely, a fee-for-service model may leverage existing remote billing to support a program. Remote care programs offer several potential value levers that will vary depending on the local health care and payer environment.
Limitations
The program was performed as a prospective quality improvement program that implemented guideline-directed medical care. Because of the nonrandomized nature of our intervention, it is impossible to prove a causal relationship between intervention and outcomes; however, our population was large, diverse, and spread across many different clinical settings and included patients who had been in our system for many years without optimization of their BP or cholesterol. Moreover, the results were consistent in many subpopulations. The degree of improvements in BP and LDL-C appears greater than would be expected with simple regression to the mean. Similar implementation strategies have also demonstrated comparable improvements to standard of care with sustained benefit.42,43
As is often observed in clinical initiatives, many patients did not fully complete the program.48,49 Some patients who exited the program before reaching maintenance already received their initial therapeutic recommendations, so may derive benefit from a first round of medication changes. Patients incurred no extra costs for our program (including the BP cuff) except any copayments required by their insurance for medications and laboratory tests. It is unknown if any additional cost would have changed the participation or outcomes.
Conclusions
The findings in this study indicated an association between remote health delivery at scale and improvements in chronic disease metrics in a large urban and suburban outpatient cohort and across racial, ethnic, and language populations historically underserved by health care. We believe that this program may serve as a model for health care professionals and systems aiming to enhance access, patient engagement, and health outcomes.
eMethods
eTable 1. Patient Demographics in the Hypertension and Cholesterol Programs
eTable 2. Number of activities for patients to reach Maintenance
eTable 3. Rates of contact and enrollment stratified by Demographics of interest
eTable 4. Demographics of Educated-only and Medication management cohorts
eFigure 1. Hypertension Flow diagram: Broad overview (CCB – calcium-channel blocker, ARB – angiotensin receptor blocker, BB – beta-blocker)
eFigure 2. Statin Management Overview
eFigure 3. Patient Disposition in the combined Hypertension and Lipids Programs
eFigure 4. Changes in Medications During Management in patients who reach maintenance
References
- 1.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):e13-e115. doi: 10.1161/HYP.0000000000000065 [DOI] [PubMed] [Google Scholar]
- 2.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1082-e1143. doi: 10.1161/CIR.0000000000000625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bundy JD, Mills KT, Chen J, Li C, Greenland P, He J. Estimating the association of the 2017 and 2014 hypertension guidelines with cardiovascular events and deaths in US adults: an analysis of national data. JAMA Cardiol. 2018;3(7):572-581. doi: 10.1001/jamacardio.2018.1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olufade T, Zhou S, Anzalone D, et al. Initiation patterns of statins in the 2 years after release of the 2013 American College of Cardiology/American Heart Association (ACC/AHA) Cholesterol Management Guideline in a large US health plan. J Am Heart Assoc. 2017;6(5):e005205. doi: 10.1161/JAHA.116.005205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu Y, Liu Y, Dhingra LS, et al. Trends in awareness, antihypertensive medication use and blood pressure control by race and ethnicity among adults with hypertension in the United States: a national health and nutrition examination analysis from 2011 to 2018. medRxiv. Preprint posted online January 1, 2021. doi: 10.1101/2021.08.18.21262251 [DOI]
- 6.Muntner P, Hardy ST, Fine LJ, et al. Trends in blood pressure control among US adults with hypertension, 1999-2000 to 2017-2018. JAMA. 2020;324(12):1190-1200. doi: 10.1001/jama.2020.14545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rethy L, Shah NS, Paparello JJ, Lloyd-Jones DM, Khan SS. Trends in hypertension-related cardiovascular mortality in the United States, 2000 to 2018. Hypertension. 2020;76(3):e23-e25. doi: 10.1161/HYPERTENSIONAHA.120.15153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Superko HR, Williams PT, Dansinger M, Schaefer E. Trends in low-density lipoprotein-cholesterol blood values between 2012 and 2017 suggest sluggish adoption of the recent 2013 treatment guidelines. Clin Cardiol. 2019;42(1):101-110. doi: 10.1002/clc.23115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown AF, Liang LJ, Vassar SD, et al. Trends in racial/ethnic and nativity disparities in cardiovascular health among adults without prevalent cardiovascular disease in the United States, 1988 to 2014. Ann Intern Med. 2018;168(8):541-549. doi: 10.7326/M17-0996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuehn BM. Hypertension rates in rural areas outpace those in urban locales. JAMA. 2020;323(24):2454. doi: 10.1001/jama.2020.9382 [DOI] [PubMed] [Google Scholar]
- 11.Aggarwal R, Chiu N, Wadhera RK, et al. Racial/ethnic disparities in hypertension prevalence, awareness, treatment, and control in the United States, 2013 to 2018. Hypertension. 2021;78(6):1719-1726. doi: 10.1161/HYPERTENSIONAHA.121.17570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al Kibria GM. Racial/ethnic disparities in prevalence, treatment, and control of hypertension among US adults following application of the 2017 American College of Cardiology/American Heart Association guideline. Prev Med Rep. 2019;14:100850. doi: 10.1016/j.pmedr.2019.100850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim EJ, Kim T, Paasche-Orlow MK, Rose AJ, Hanchate AD. Disparities in hypertension associated with limited English proficiency. J Gen Intern Med. 2017;32(6):632-639. doi: 10.1007/s11606-017-3999-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.US Department of Health and Human Services . Hypertension Cascade: Hypertension Prevalence, Treatment and Control Estimates Among US Adults Aged 18 Years and Older Applying the Criteria From the American College of Cardiology and American Heart Association’s 2017 Hypertension Guideline—NHANES 2015–2018. Accessed March 20, 2022. https://millionhearts.hhs.gov/data-reports/hypertension-prevalence.html
- 15.Desai AS, Maclean T, Blood AJ, et al. Remote optimization of guideline-directed medical therapy in patients with heart failure with reduced ejection fraction. JAMA Cardiol. 2020;5(12):1430-1434. doi: 10.1001/jamacardio.2020.3757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Plutzky J, Benson MD, Chaney K, et al. Population health management of low-density lipoprotein cholesterol via a remote, algorithmic, navigator-executed program. Am Heart J. 2022;243:15-27. doi: 10.1016/j.ahj.2021.08.017 [DOI] [PubMed] [Google Scholar]
- 17.Fisher NDL, Fera LE, Dunning JR, et al. Development of an entirely remote, non-physician led hypertension management program. Clin Cardiol. 2019;42(2):285-291. doi: 10.1002/clc.23141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scirica BM, Cannon CP, Fisher NDL, et al. Digital care transformation: interim report from the first 5000 patients enrolled in a remote algorithm-based cardiovascular risk management program to improve lipid and hypertension control. Circulation. 2021;143(5):507-509. doi: 10.1161/CIRCULATIONAHA.120.051913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grossman LV, Masterson Creber RM, Benda NC, Wright D, Vawdrey DK, Ancker JS. Interventions to increase patient portal use in vulnerable populations: a systematic review. J Am Med Inform Assoc. 2019;26(8-9):855-870. doi: 10.1093/jamia/ocz023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khoong EC, Rivadeneira NA, Hiatt RA, Sarkar U. The use of technology for communicating with clinicians or seeking health information in a multilingual urban cohort: cross-sectional survey. J Med internet Res. 2020;22(4):e16951. doi: 10.2196/16951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nouri S, Khoong E, Lyles C, Karliner L. Addressing equity in telemedicine for chronic disease management during the COVID-19 pandemic. NEJM Catalyst . Published May 4, 2020. Accessed September 21, 2021. https://catalyst.nejm.org/doi/full/10.1056/CAT.20.0123
- 22.Merid B, Robles MC, Nallamothu BK. Digital redlining and cardiovascular innovation. Circulation. 2021;144(12):913-915. doi: 10.1161/CIRCULATIONAHA.121.056532 [DOI] [PubMed] [Google Scholar]
- 23.Gordon WJ, Blood AJ, Chaney K, et al. Workflow automation for a virtual hypertension management program. Appl Clin Inform. 2021;12(5):1041-1048. doi: 10.1055/s-0041-1739195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults. Circulation. 2014;129(25_suppl_2). doi: 10.1161/01.cir.0000437738.63853.7a [DOI] [PubMed] [Google Scholar]
- 25.Collaborative drug therapy management. M.G.L. c. 112, § 24B1/2; 2017:1-4.
- 26.Koonin LM, Hoots B, Tsang CA, et al. Trends in the use of telehealth during the emergence of the COVID-19 pandemic—United States, January-March 2020. MMWR Morb Mortal Wkly Rep. 2020;69(43):1595-1599. doi: 10.15585/mmwr.mm6943a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weiner JP, Bandeian S, Hatef E, Lans D, Liu A, Lemke KW. In-person and telehealth ambulatory contacts and costs in a large US insured cohort before and during the COVID-19 pandemic. JAMA Netw Open. 2021;4(3):e212618. doi: 10.1001/jamanetworkopen.2021.2618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chu R, Peters C, de Lew N, Sommers B. State Medicaid telehealth policies before and during the COVID-19 public health emergency. Am J Public Health. Accessed April 19, 2022. https://aspe.hhs.gov/reports/state-medicaid-telehealth-policies
- 29.Centers for Medicare & Medicaid Services . COVID-19 emergency declaration blanket waivers for health care providers. Accessed April 19, 2022. https://www.cms.gov/files/document/covid-19-emergency-declaration-waivers.pdf
- 30.Pike S. How Americans feel about telehealth: one year later. SYKES . Accessed March 22, 2022. https://www.sykes.com/resources/reports/how-americans-feel-about-telehealth-now/
- 31.Bestsennyy O, Gilbert G, Harris A, et al. Telehealth: a post-COVID-19 reality? McKinsey . Accessed April 4, 2022. https://www.mckinsey.com/industries/healthcare-systems-and-services/our-insights/telehealth-a-quarter-trillion-dollar-post-covid-19-reality
- 32.Walia B, Shridhar A, Arasu P, Singh GKUS. US physicians’ perspective on the sudden shift to telehealth: survey study. JMIR Hum Factors. 2021;8(3):e26336. doi: 10.2196/26336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reed ME, Huang J, Graetz I, et al. Patient characteristics associated with choosing a telemedicine visit vs office visit with the same primary care clinicians. JAMA Netw Open. 2020;3(6):e205873. doi: 10.1001/jamanetworkopen.2020.5873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mehrotra A, Chernew M, Linetsky D, Hatch H, Cutler D, Schneider E. The impact of COVID-19 on outpatient visits in 2020: visits remained stable, despite a late surge in cases. Commonwealth Fund . Accessed April 19, 2022. https://www.commonwealthfund.org/publications/2021/feb/impact-covid-19-outpatient-visits-2020-visits-stable-despite-late-surge
- 35.Telemedicine Market Size, Share, & COVID-19 Impact Analysis. Fortune Business Insights . Accessed April 19, 2022. https://www.fortunebusinessinsights.com/industry-reports/telemedicine-market-101067
- 36.Camlek V. Telehealth—a technology-based weapon in the war against the coronavirus. Frost & Sullivan . Accessed April 19, 2022. https://wnydocs.org/resources/Documents/TelehealthA%20Technology-Based%20Weapon%20in%20the%20War%20Against%20the%20Coronavirus%202020.pdf
- 37.Choi H, Steptoe A, Heisler M, et al. Comparison of health outcomes among high- and low-income adults aged 55 to 64 years in the US vs England. JAMA Intern Med. 2020;180(9):1185-1193. doi: 10.1001/jamainternmed.2020.2802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schanzenbach D, Mumford M, Nunn R, Bauer L. Money lightens the load. The Hamilton Project . Accessed April 19, 2022. https://www.hamiltonproject.org/papers/money_lightens_the_load
- 39.Williams DR, Priest N, Anderson NB. Understanding associations among race, socioeconomic status, and health: patterns and prospects. Health Psychol. 2016;35(4):407-411. doi: 10.1037/hea0000242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.U.S. Census Bureau QuickFacts . Massachusetts. Accessed March 12, 2022. https://www.census.gov/quickfacts/MA
- 41.Margolis KL, Dehmer SP, Sperl-Hillen J, et al. . Cardiovascular events and costs with home blood pressure telemonitoring and pharmacist management for uncontrolled hypertension. Hypertension. 2020;76(4):1097-1103. doi: 10.1161/HYPERTENSIONAHA.120.15492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Margolis KL, Asche SE, Bergdall AR, et al. Effect of home blood pressure telemonitoring and pharmacist management on blood pressure control: a cluster randomized clinical trial. JAMA. 2013;310(1):46-56. doi: 10.1001/jama.2013.6549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mills KT, Obst KM, Shen W, et al. Comparative effectiveness of implementation strategies for blood pressure control in hypertensive patients: a systematic review and meta-analysis. Ann Intern Med. 2018;168(2):110-120. doi: 10.7326/M17-1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Margolis KL, Asche SE, Dehmer SP, et al. Long-term outcomes of the effects of home blood pressure telemonitoring and pharmacist management on blood pressure among adults with uncontrolled hypertension: follow-up of a cluster randomized clinical trial. JAMA Netw Open. 2018;1(5):e181617-e181617. doi: 10.1001/jamanetworkopen.2018.1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blyler CA, Ebinger J, Rashid M, et al. Improving efficiency of the barbershop model of hypertension care for Black men with virtual visits. J Am Heart Assoc. 2021;10(13):e020796. doi: 10.1161/JAHA.120.020796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Victor RG, Lynch K, Li N, et al. A cluster-randomized trial of blood-pressure reduction in Black barbershops. N Engl J Med. 2018;378(14):1291-1301. doi: 10.1056/NEJMoa1717250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pratap A, Neto EC, Snyder P, et al. . Indicators of retention in remote digital health studies: a cross-study evaluation of 100,000 participants. NPJ Digit. 2020;3(1):1-10. doi: 10.1038/s41746-020-0224-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goldman AI, Holcomb R, Perry HM Jr, Schnaper HW, Fitz AE, Frohlich ED. Can dropout and other noncompliance be minimized in a clinical trial? report from the Veterans Administrative National Heart, Lung and Blood Institute cooperative study on antihypertensive therapy: mild hypertension. Control Clin Trials. 1982;3(2):75-89. doi: 10.1016/0197-2456(82)90037-X [DOI] [PubMed] [Google Scholar]
- 49.Atlantis E, Chow CM, Kirby A, Fiatarone Singh MA. Worksite intervention effects on physical health: a randomized controlled trial. Health Promot Int. 2006;21(3):191-200. doi: 10.1093/heapro/dal012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
eTable 1. Patient Demographics in the Hypertension and Cholesterol Programs
eTable 2. Number of activities for patients to reach Maintenance
eTable 3. Rates of contact and enrollment stratified by Demographics of interest
eTable 4. Demographics of Educated-only and Medication management cohorts
eFigure 1. Hypertension Flow diagram: Broad overview (CCB – calcium-channel blocker, ARB – angiotensin receptor blocker, BB – beta-blocker)
eFigure 2. Statin Management Overview
eFigure 3. Patient Disposition in the combined Hypertension and Lipids Programs
eFigure 4. Changes in Medications During Management in patients who reach maintenance