FIGURE 1.
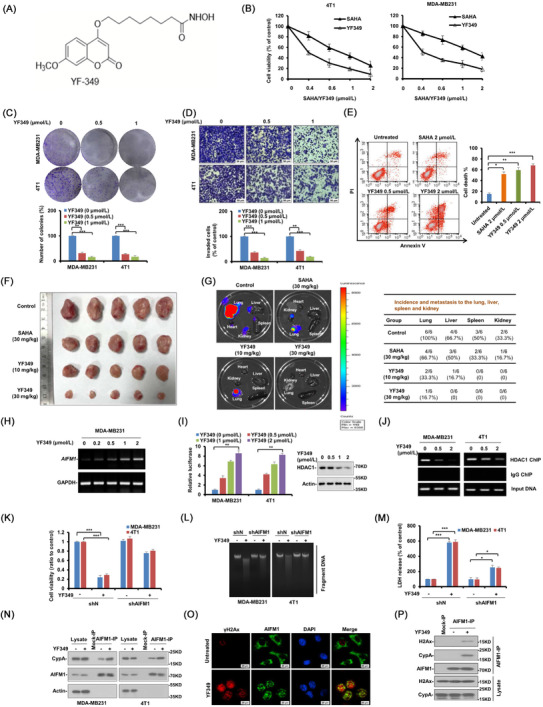
YF349 exerts promising anti‐breast cancer activity by triggering AIFM1‐dependent programmed necrosis. (A) Chemical structure of the new HDACI, YF349. (B) Breast cancer cells (MDA‐MB231 and 4T1) were treated with YF349 or SAHA. After 48 h, MTS assay was performed. The bars indicate mean ± SD. (C) MDA‐MB231 and 4T1 cells were seeded on 6‐well plates. After 12 h, cells were treated with indicated concentrations of YF349. On day 10, the number of colonies was counted in experiments repeated three times. Results represent the average of three replications. (D) MDA‐MB231 and 4T1 cells were treated with different concentrations of YF349 and allowed to invade through matrigel. Images were obtained after 12 h of incubation (upper). The invaded cell number was counted and expressed as % untreated control (lower). Data were shown as mean ± SD from three independent experiments. Scale: 50 μm. (E) 4T1 cells were treated with different dosages of YF349 or SAHA for 48 h. Cell death was assessed by Annexin V/PI staining and flow cytometry. (F) MDA‐MB231‐luc cells (1×105) were injected into the mammary fat pad of nude mice. Mice were divided into 5 groups (n = 5 per group) on day 7. Mice were administrated with YF349 or SAHA every day. After 35 days, all mice were sacrificed. (G) Ex vivo bioluminescence images were obtained in each group to determine the effects of YF349 against distant metastasis. Metastasis in distant organs was quantified. (H) MDA‐MB231 cells were treated with YF349 with the indicated concentrations for 24 h, RNA samples were prepared using Trizol and total RNA was converted to cDNA using oligodT primer. The relative expression of AIFM1 was analysed by RT‐PCR with GAPDH as an internal control. PCR products were separated on 1.2% agarose gel and stained with ethidium bromide. (I) Human AIFM1 promoter (1343 to 141 bp upstream of ATG) luciferase reporter was transfected in MDA‐MB231 and 4T1 cells, and 12 h later, the cells were treated with increased concentrations of YF349 for 24 h, and then the relative luciferase activity was analyzed. Data were presented as mean ± SD, ** P < 0.01. Representative western blotting shows the expression of HDAC1 and Actin. (J) YF349 blocks HDAC1 binding to the AIFM1 promoter. MDA‐MB231 and 4T1 cells were treated with YF349 or not, and cells were processed for chromatin immunoprecipitation using HDAC1 antibody. Co‐precipitated chromatin DNA was analyzed by PCR using a pair of primers that amplify the 463 to 318 bp region of the AIFM1 promoter. (K) Cell viability was analyzed using MTS assay in AIFM1‐knockdown cells under YF349 treatment or not (*** P < 0.001). (L) The large‐scale DNA fragmentations in AIFM1‐knockdown cells treated with YF349 or not were detected on agarose gel electrophoresis. (M) The LDH release assay was performed in AIFM1‐knockdown cells under YF349 treatment or not (*** P < 0.001). (N) MDA‐MB231 and 4T1 cells were treated with YF349 for 24 h, and the co‐localization of AIFM1 and CypA was examined by immunoprecipitation assay. (O) 4T1 cells were treated with 2 μmol/L YF349 for 12 h and then washed with PBS three times. AIFM1 (green), γH2AX (red) and DAPI (blue) were detected by immunofluorescence staining (scale bar 20 μm). (P) MDA‐MB231 cells treated with or without YF349 were lysed and immunoprecipitated using AIFM1 antibody followed by anti‐H2AX and anti‐CypA western blot.
