SUMMARY
Rigosertib is a styryl benzyl sulfone that inhibits growth of tumor cells and acts as a RAS mimetic by binding to Ras binding domains of RAS effectors. A recent study attributed rigosertib’s mechanism of action to microtubule binding. In that study, rigosertib was obtained from a commercial vendor. We compared the purity of clinical-grade and commercially sourced rigosertib and found that commercially sourced rigosertib contains approximately 5% ON01500, a potent inhibitor of tubulin polymerization. Clinical-grade rigosertib, which is free of this impurity, does not exhibit tubulin-binding activity. Cell lines expressing mutant β-tubulin have also been reported to be resistant to rigosertib. However, our study showed that these cells failed to proliferate in the presence of rigosertib at concentrations that are lethal to wild-type cells. Rigosertib induced a senescence-like phenotype in the small percentage of surviving cells, which could be incorrectly scored as resistant using short-term cultures.
In Brief
A recent study using commercially available rigosertib attributed its mechanism of action to microtubule binding. Baker et al. find that commercially sourced rigosertib contains an impurity, ON01500, that inhibits tubulin polymerization. Clinical-grade rigosertib is devoid of this impurity and does not bind to tubulin.
Graphical Abstract
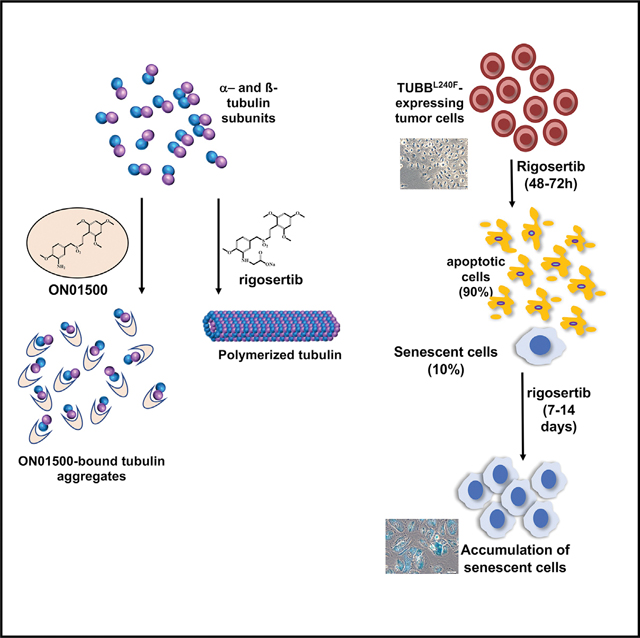
INTRODUCTION
Rigosertib is a novel styryl benzyl sulfone that inhibits growth of a wide variety of human tumor cells in vitro and impairs tumor growth in vivo with little toxicity (Jimeno et al., 2009; Reddy et al., 2011; Agoni et al., 2014; Silverman et al., 2015). We recently described the mechanism of action of rigosertib (Athuluri-Divakar et al., 2016) and provided structural and biochemical evidence to show that rigosertib acts as a RAS mimetic and binds to the Ras binding domains (RBDs) of the RAF and phosphatidylinositol 3-kinase (PI3K) family proteins and disrupts their ability to bind to RAS. This conclusion was based on a number of biochemical assays that included chemical pull-down of target proteins by rigosertib bound to agarose beads, thermal shift assays, microscale thermophoresis (MST), and nuclear magnetic resonance (NMR) spectroscopy to derive the structure of the rigosertib-RAF-RBD complex. We also demonstrated that this compound inhibits RAS-mediated activation of the mitogen-activated protein kinase (MAPK) and protein kinase B (AKT) pathways in vitro as well as in animal models of RAS-induced tumors.
In a recent study, Jost et al. (2017) reported microtubule-binding activity of rigosertib, which was purchased from a commercial vendor. In the binding studies, Jost et al. (2017) reported that rigosertib, at high micromolar concentrations (>20 μM), showed microtubule-depolymerizing activity. Because we were unable to reproduce the results of Jost et al. (2017), and impurities present in non-clinical-grade preparations of rigosertib could have a significant effect on binding results, we examined the chemical and molecular basis of this discrepancy. We found that commercial-grade preparations of rigosertib are often contaminated with synthetic intermediates and degradation products of rigosertib that contribute to the tubulin-depolymerizing activity of these preparations. In addition, our results showed that wild-type β class tubulin (TUBB) and TUBB L240F-expressing cells failed to proliferate in the presence of clinical-grade rigosertib at concentrations that are lethal to wild-type cells. We also found that rigosertib, at lethal concentrations, induced a senescence-like phenotype in small percentages of wild-type and TUBB L240F-expressing cells and that these senescent cells could be incorrectly scored as resistant cells in flow cytometry assays using short-term cultures.
RESULTS
Commercial Preparations of Rigosertib Are Often Contaminated with ON01500, an Intermediate with Potent Tubulin-Depolymerizing Activity
A key late-stage intermediate in synthesis of rigosertib is ON01500 (Figure 1A), an amino styryl benzyl sulfone with potent tubulin-binding and depolymerization activity. Conversion of the amino group of ON01500 into a glycyl moiety results in formation of rigosertib (ON01910) (Reddy et al., 2011). Because the two compounds have distinct physiochemical properties (such as aqueous solubility), final purification and quality control measures are incorporated in the current good manufacturing practice (cGMP) manufacturing of rigosertib, which is conducted under contract by the company (Onconova Therapeutics) conducting clinical trials of this compound. A typical batch of clinical material is 99.9% pure and could include 0.1% of these impurities. Several storage conditions, including higher temperature, acidic pH, and exposure to intense light can lead to degradation of rigosertib into ON01500, as shown in Figure 1B (Patel et al., 2018 and data not shown). We compared several batches of material obtained from the commercial vendor (Selleckchem) with materials obtained directly from Onconova Therapeutics.
Figure 1. Purity and Tubulin-Binding Activities of Pharmaceutical-Grade and Commercial-Grade Rigosertib.
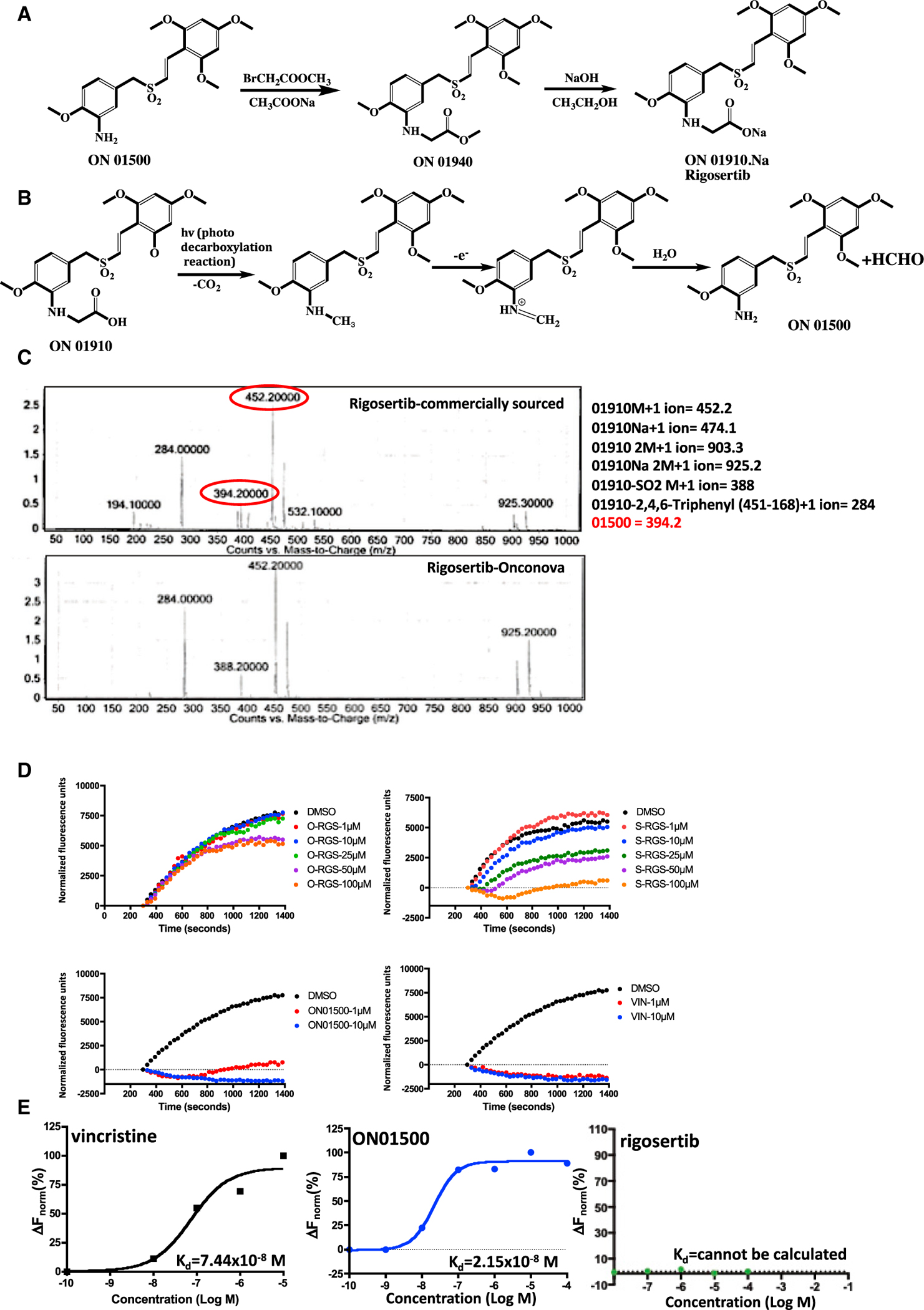
(A) Synthetic scheme used for preparation of rigosertib (Reddy et al., 2011).
(B) Photodegradation of rigosertib to ON01500.
(C) LC-MS/MS analysis of rigosertib obtained from Onconova Therapeutics and Selleckchem.
(D) Polymerization of tubulin in the presence of rigosertib from Onconova Therapeutics (O-RGS) and Selleckchem (S-RGS), ON01500 (Onconova Therapeutics), and vincristine (VIN). 25 μg of MAP-rich tubulin in general tubulin buffer was mixed with 1 mM guanosine triphosphate (GTP) and fluorescence reporter in the presence of vehicle (DMSO) or increasing concentrations of the indicated compound. Tubulin polymerization as a function of fluorescence was recorded over the indicated time at 37°C.
(E) Microscale thermophoretic analysis of ON01500 and VIN with purified tubulin. Tubulin was labeled using the Monolith NT protein labeling kit RED-NHS according to the instructions of the manufacturer. Labeled protein was incubated with increasing concentrations of rigosertib, ON01500, or VIN for 30 min and subjected to MST.
To determine whether these preparations of rigosertib are contaminated with ON01500, we analyzed both preparations by NMR and mass spectrometry. The results of one such study are presented in Figure 1C and show that this particular batch of rigosertib purchased from Selleckchem contained approximately 5% of ON01500 as well as a few additional contaminants, whereas clinical-grade rigosertib obtained from Onconova Therapeutics had undetectable amounts of these contaminants.
Next we compared the tubulin-depolymerizing activities of clinical-grade rigosertib provided by Onconova Therapeutics (O-RGS), commercial-grade rigosertib purchased from Selleckchem (S-RGS), and ON01500. Vincristine was used as a positive control. Tubulin polymerization assays were performed using microtubule-associated protein (MAP)-rich tubulin and a fluorescence-based dye that, when incorporated into polymerizing tubulin, results in fluorescence enhancement as polymerization occurs. The results of this study, presented in Figure 1D, show that the highly purified preparation of O-RGS shows little or no tubulin-depolymerizing activity at doses of up to 50 μM. We observed a delay in tubulin polymerization at concentrations of 50 μM and 100 μM, which could be attributed to the contaminants, which are less than 0.1%. These concentrations are ~500–1,000-fold higher than the GI50 value of rigosertib for any tumor cell line that is sensitive to the effects of this compound. In contrast, tubulin was completely depolymerized in the presence of as little as 1 μM ON01500 or vincristine. S-RGS, on the other hand, exhibits depolymerizing activity when used at concentrations above 25 μM, reaching complete depolymerization at a concentration of 100 μM (Figure 1D), suggestive of the effect of the impurity.
Next we examined the binding affinity of pure ON01500, O-RGS, and vincristine to purified tubulin preparations using MST (Wienken et al., 2010). The results of this study, shown in Figure 1E, demonstrate that vincristine and ON01500 bind to tubulin with similar high affinities, which are reflected in their dissociation constants of 74 and 21 nM, respectively. When we examined binding of O-RGS using this technique, we were unable to detect binding, even at a concentration of 100 μM.
Crystallography Studies Using Commercial-Grade Rigosertib with β-Tubulin
Jost et al. (2017) also report the crystal structure of tubulin in complex with commercially sourced rigosertib. Figure 2 shows the 2Fo-Fc electron density for the modeled rigosertib (PDB: 5OV7) displayed at 0.5σ and 0.8σ, as well as at the more conventional 1σ cutoffs. The electron density for the carboxyl group is relatively weak, and the proposed hydrogen bonding interactions with αS178 (distances of 3.9 and 3.0 Å for molecules B and D, respectively) and ßLys352 (5.1 and 4.2 Å, respectively) are less than optimal. In our opinion, a more likely interpretation of the weak electron density would be that a water molecule with the capacity to establish hydrogen bonding interactions with the side chain of ßAsn349 is occupying this space. In light of our data showing the presence ON01500 in commercially sourced rigosertib and the capacity of ON01500 to bind and depolymerize tubulins, the proposed structure is arguably more consistent with the binding of ON01500 and a water molecule rather than rigosertib to tubulin.
Figure 2. 2Fo-Fc Electron Density Map (Gray) for Rigosertib Modeled in the Two αβ-Tubulin Heterodimers in the Asymmetric Unit of PDB: 5OV7.
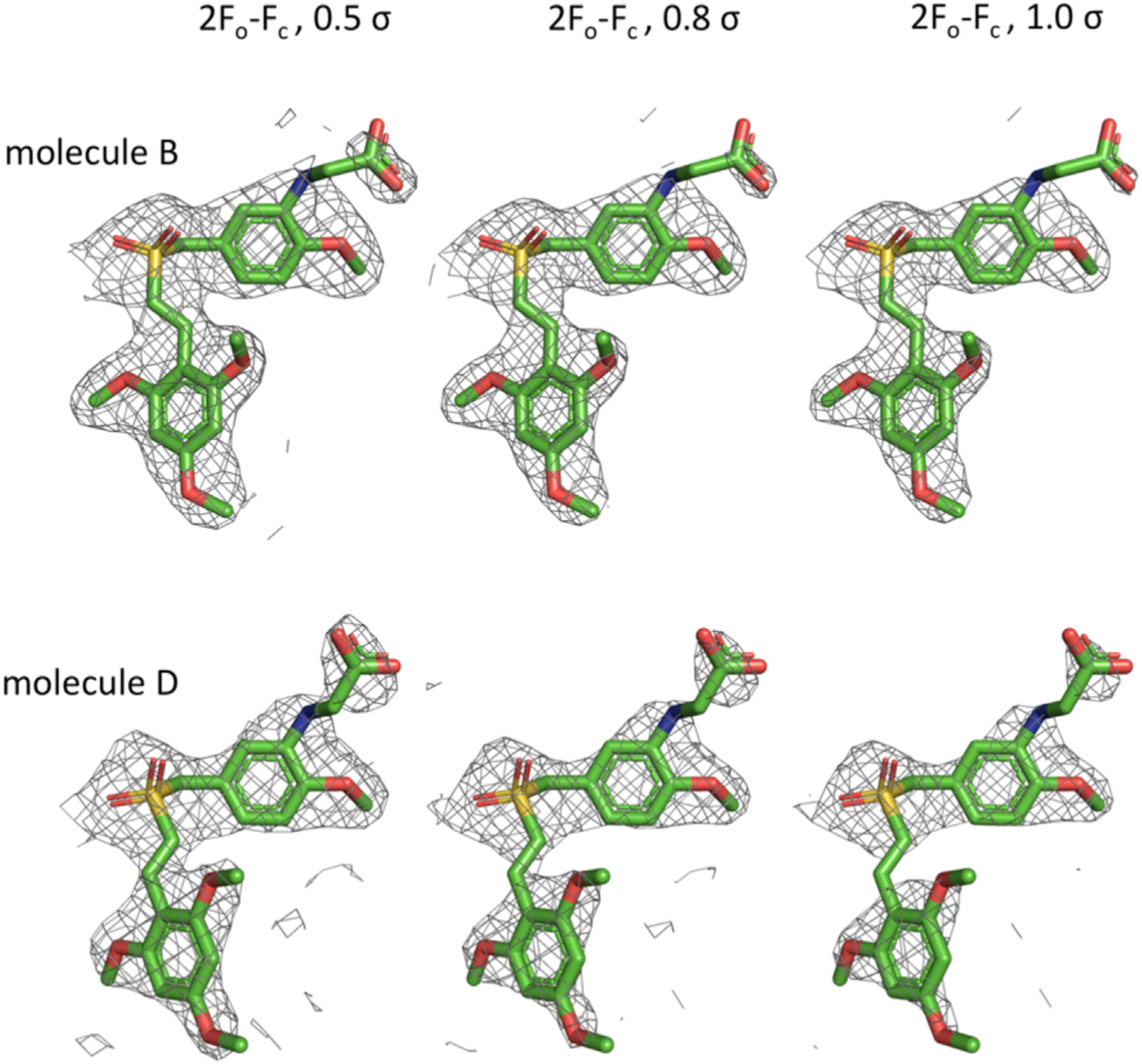
Density is contoured at 0.5σ (left), 0.8s (middle), and at 1σ (right).
The L240F Tubulin Mutant Does Not Confer Resistance to Rigosertib
Jost et al. (2017) reported that expression of the L240F β-tubulin mutant provides resistance to rigosertib, suggesting that tubulin binding is critical for its cytotoxic activity. For these experiments, Jost et al. (2017) prepared lentiviral constructs that encoded an empty vector, wild-type (WT) TUBB or TUBB L240F, which were individually transduced into WT K562, HeLa, or H358 cells. These cell lines, which express the mCherry marker, were combined at a 1:1 ratio with their respective parental lines and treated with rigosertib or DMSO, and the fraction of TUBB-expressing cells was measured up to 7 days after treatment as mCherry-positive cells by flow cytometry. An elevated ratio of mCherry-expressing cells after rigosertib treatment compared with DMSO was interpreted to indicate that expression of L240F mutant tubulin confers resistance. To examine this phenomenon in greater detail, we obtained lentiviral vectors that encode WT tubulin or the L240F β-tubulin mutant from Dr. Weissman’s laboratory and repeated these studies using K562 cells that were transduced with an empty vector or the TUBB L240F expression vector. Seventy-two hours post-infection, the cells were mixed 1:1 with uninfected cells and treated with DMSO, increasing concentrations of rigosertib or BI2536, a pan-PLK (polo-like kinase) inhibitor, as a control. Growth curves of the mCherry+ cells that express TUBB L240F and of mCherry− cells (uninfected controls) are shown in Figure 3A. The results of this study show that mCherry+ cells that express TUBB-L240F or the empty vector and mCherry− cells that represent parental cells are inhibited by rigosertib as well as BI2536 in a concentration-dependent manner with very similar kinetics. Next we repeated these studies and measured the effect of rigosertib and BI2536 on the viability of K562 cells that express TUBB L240F or an empty vector. The results of this study, shown in Figure 3B, demonstrate that rigosertib and BI2516 induce cell death in mCherry+, TUBB L240F-expressing cells and parental (mCherry−) cells with similar kinetics. This effect was also seen with empty vector-expressing cells and their parental counterparts. Cells treated with rigosertib at concentrations of 100 or 200 nM had less than 10% viable cells on day 6 compared with their untreated controls, suggesting that expression of mutant TUBB had no effect on the growth-inhibitory or apoptosis-inducing activities of rigosertib or BI2536.
Figure 3. Expression of Mutant β-Tubulin Does Not Confer a Significant Survival Advantage in K562 Cells Treated with Rigosertib.
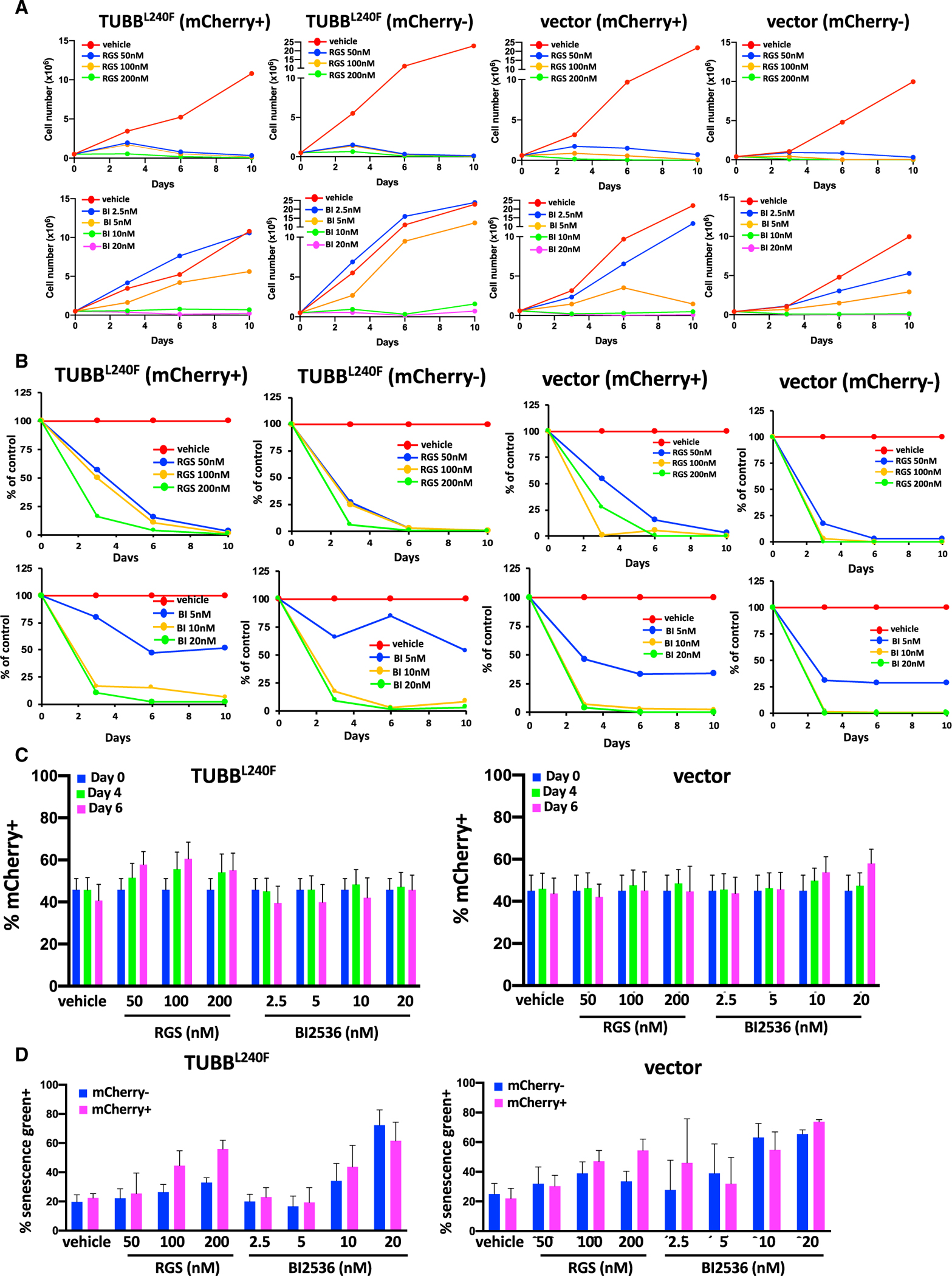
(A and B) Treatment with rigosertib induces a dose-dependent decrease in (A) proliferation and (B) viability of mCherry-positive TUBBL240F and WT K562 cells over time. K562 cells were infected with lentiviruses encoding β-tubulin L240F or an empty vector and combined with WT (mCherry−) cells at a final density of 1.0 × 105 cells/mL. The cells were then treated with increasing concentrations of rigosertib, BI2536, or vehicle (DMSO) and harvested on days 1, 4, and 6. The percentages of viable (DAPI−) mCherry+ cells were determined using flow cytometric analysis.
(C) Percentage of mCherry+ K562 cells expressing TUBBL240F as a function of time. K562 cells were infected with lentiviruses encoding mutant L240F β-tubulin or a vector control for 24 h. The percentage of mCherry+ cells was determined by flow cytometry analysis 48 h post-infection, and the cells were combined with WT (mCherry−) cells at a final density of 1.0 × 105 cells/mL. The cells were then treated with increasing concentrations of rigosertib, BI2536, or vehicle (DMSO). Cells were harvested on days 4 and 6, and the percentage of viable (DAPI−) mCherry+ cells was determined using flow cytometric analysis. Error bars represent mean ± SEM.
(D) Treatment with rigosertib induces senescence in WT and TUBBL240F K562 cells. K562 cells were infected with lentiviruses as described in (A) and grown in the presence of increasing concentrations of rigosertib or BI2536 for a 7-day period. mCherry+ and mCherry− cells were isolated using fluorescence-activated cell sorting and fixed, and the level of β-galactosidase was determined by staining with CellEvent senescence green and subsequent flow cytometric analysis. Data are represented as mean ± SEM.
When we examined the ratio of mCherry+ cells in the small viable fraction that remained at days 4 and 6, we observed a slightly higher fraction of TUBB L240F-expressing cells in rigosertib-treated cells (Figure 3C). The ratio of mCherry+ cells in vehicle-treated cultures was approximately 50% on days 0, 4, and 6, and this ratio increased to 65% to 70% in cells that express TUBB L240F. Although this increase was not statistically significant, this trend was seen repeatedly in multiple experiments. This observation is consistent with that made by Jost et al. (2017), who interpreted this population to be rigosertib-resistant cells. However, when we examined the surviving cells under the microscope, they were unusually large in size with a cellular morphology that is characteristic of senescent cells. We therefore examined the surviving population for increased levels of β-galactosidase, one of the most reliable markers of senescence. These analyses demonstrated that the majority of the remaining cells in all cultures, including those that express TUBB L240F, exhibited increased β-galactosidase levels, as demonstrated by traditional staining (data not shown) as well as flow cytometry analysis using CellEvent senescence green (Figure 3D), confirming that the remaining cells scored positive for this senescence marker (Figure 3D). In spite of prolonged incubation in growth medium, we were unable to establish cell cultures that can grow in the presence of rigosertib, suggesting that this residual population of cells was truly senescent and does not represent rigosertib-resistant cells.
To further confirm this observation and extend the studies, we developed several stably transfected clonal cell lines that express mutant β-tubulin. We chose to use K562 as well as A549 cells, which are KRAS mutant lung carcinoma cells that have been shown by us to be sensitive to rigosertib-mediated growth inhibition in vitro and in vivo (Athuluri-Divakar et al., 2016). For these studies, lentiviral constructs that encoded an empty vector, WT TUBB, or TUBB L240F were individually transduced into K562 and A549 cells, and clones that stably expressed TUBB L240 and the mCherry vector were selected using limiting dilution. Clones that were more than 95% mCherry+ by flow cytometric analysis were selected for further study, with representative K562 and A549 clones shown in Figures 4A and 5A (left panels). To determine whether L240F TUBB confers resistance to rigosertib, exponentially growing K562 clones were grown in the presence of increasing concentrations of vehicle or rigosertib for a period of 96 h. At the end of the study, viability was determined using CellTiter Blue. The results of this study (Figure 4A, right panel) show that expression of the L240F β-tubulin mutant does not confer a significant survival advantage to this cell line.
Figure 4. Expression of L240F Mutant β-Tubulin Does Not Confer Resistance to Rigosertib.
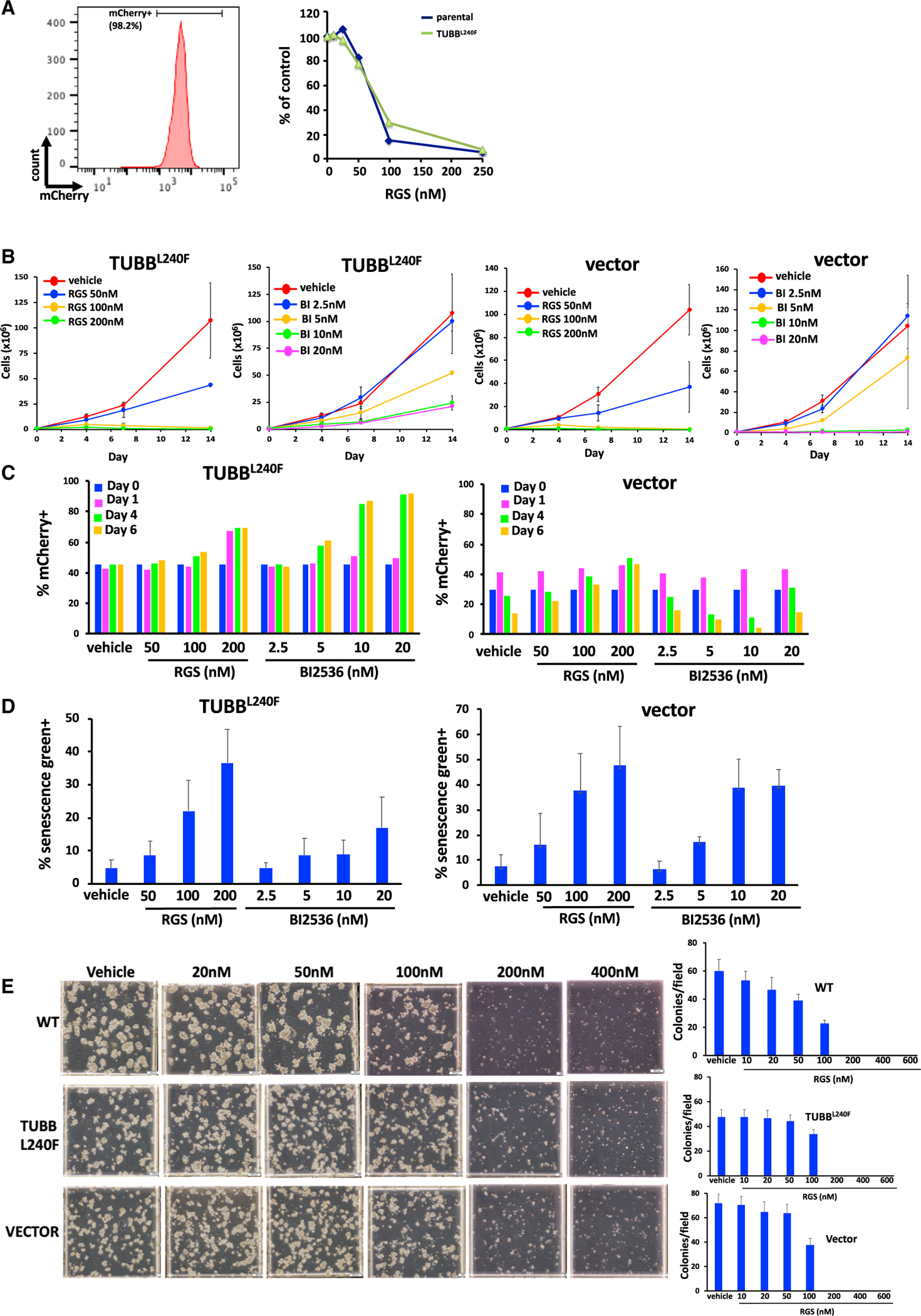
K562 cells were infected with a lentivirus that encodes the L240F mutant form of β-tubulin along with the mCherry fluorescence marker, and stable cell lines were isolated using limiting dilution cloning.
(A) Flow cytometric analysis of mCherry expression in a representative K562 clone showing that more than 95% of cells are mCherry+ (left panel). These cells were then treated with the indicated concentrations of rigosertib, and the percentage of viable cells was determined 96 h after plating. Expression of the L240F β-tubulin mutant confers little or no resistance to rigosertib (right panel).
(B) Growth of L240F β-tubulin-expressing K562 cells in the presence of increasing concentrations of rigosertib or BI2536, showing that rigosertib inhibits proliferation of K562 cells that express TUBBL240F. Analysis of one representative clone of each cell line is shown. Error bars represent mean ± SEM.
(C) K562 cells expressing TUBBL240F were combined with WT cells and seeded at a density of 1 × 105 cells/mL. The cells were treated with the indicated concentrations of rigosertib or BI2536 over a 6-day period, and the percentage of mCherry+ cells was determined by flow cytometry. The percentage of mCherry+ cells also increases in the presence of BI2536, indicating that this is not a rigosertib-specific phenomenon.
(D) Treatment of K562 cells expressing TUBBL240F or a control vector with rigosertib or BI2536 induces senescence. Cells were treated with the indicated concentrations of rigosertib or BI2536 for a 7-day period, and the level of β-galactosidase activity in viable cells was measured by flow cytometric analysis using CellEvent senescence green. Error bars represent mean ± SEM.
(E) 3D growth of L240F β-tubulin-expressing and control K562 cell lines in the presence of increasing concentrations of rigosertib, showing that rigosertib inhibits their proliferation in methylcellulose. Representative images (left panel) and average quantitation of 10 fields per plate in duplicate (right panels) are shown. Data are represented as mean ± SEM.
Figure 5. Expression of Mutant β-Tubulin Does Not Induce Resistance to Rigosertib in A549 Cells.
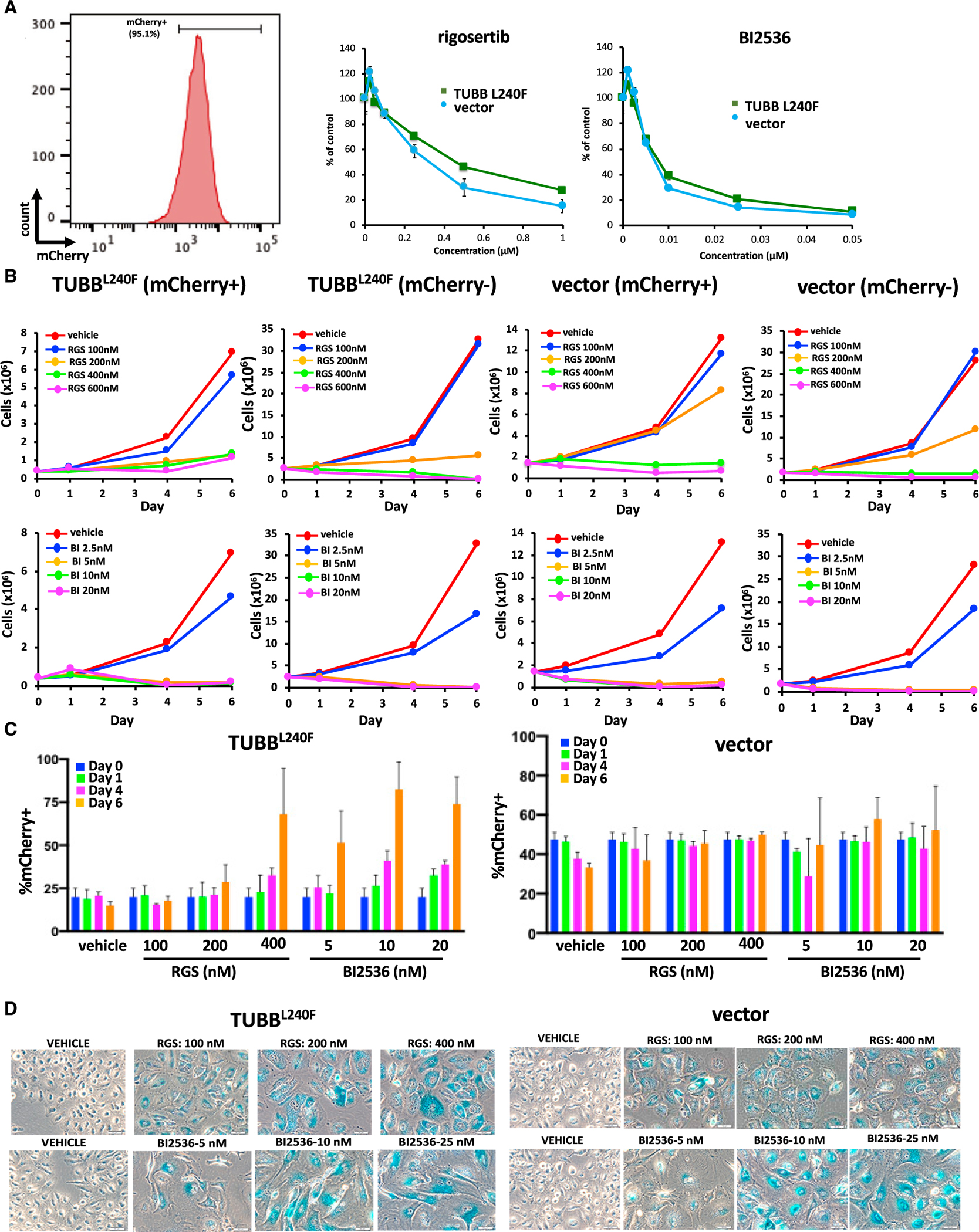
A549 cells were infected with a lentivirus that encodes the L240F mutant form of β-tubulin along with the mCherry fluorescence marker.
(A) Flow cytometry analysis of mCherry expression in representative A549 clones that are more than 95% mCherry+ (left panel). The cells were then treated with the indicated concentrations of rigosertib or BI2536, and their viability was determined 96 h post-plating (right panel). Error bars represent mean ± SEM.
(B) A549 cells expressing TUBBL240F or a vector control were combined with WT A549 cells, grown in the presence of increasing concentrations of rigosertib or BI2536, and the growth of mCherry+ and mCherry− cells was measured over a 6-day period. As observed with K562 cells, rigosertib inhibits proliferation and survival of A549 cells that express TUBBL240F. Error bars represent mean ± SEM.
(D) Prolonged treatment with rigosertib and BI2536 induces senescence in A549 cells. A549 cells expressing TUBB L240F or control cells were seeded at a density of 2 × 105 cells per well in a 6-well dish. The cells were then treated with the indicated concentrations of rigosertib or BI2536 24 h post-plating. The medium was changed weekly. After 16 days in culture, the cells were washed with PBS, fixed, and stained overnight with X-gal (0.1 mg/mL) at 37°C. Data are represented as mean ± SEM.
To compare the relative sensitivity of TUBB L240F-expressing cells with that of parental WT cells, the cells were mixed 1:1 with uninfected cells and treated with DMSO, rigosertib, or BI2536. Growth curves of the mCherry+ cells that stably express TUBB L240F and vector control are shown in Figure 4B. Cell cultures expressing L240 mutant tubulin as well as empty vector-expressing cells that were treated with rigosertib at concentrations of 100 or 200 nM had fewer than 10% viable cells remaining at day 6. When we examined the ratio of mCherry+ cells in this viable fraction, we observed a slightly higher fraction of TUBB L240F-expressing cells in rigosertib-treated cultures (Figure 4C). However, we also observed that this fraction of TUBB L240F cells was increased to a greater degree in BI2536-treated cultures, suggesting that this phenomenon is not unique to rigosertib. When we examined the surviving cells under the microscope, they displayed characteristics of senescent cells, such as an abnormally large size, with the majority of these surviving cells exhibiting increased β-galactosidase levels, as demonstrated by traditional staining (data not shown) as well as flow cytometric analysis using CellEvent senescence green (Figure 4D).
Next we examined the growth of WT, TUBB L240F-expressing, and empty vector-expressing cells in the presence of vehicle or rigosertib in MethoCult, which allows the growth of cells in 3D. The results of this study with parental K562 (WT) and K562 cells expressing TUBB L240F or the empty vector is shown in Figure 4E. These results show that growth of all three clonal cell lines is completely inhibited by 200 nM rigosertib, further confirming that expression of TUBBL240F does not confer a survival advantage to these cells.
Next we examined the effects of rigosertib and BI2536 on the growth and survival of clonal A549 cell lines that stably expressed L240FTUBB or an empty vector. A representative clone of A549 cells that is more than 95% mCherry+ is shown in Figure 5A, left panel. The results shown in Figure 5A, right panel demonstrate that rigosertib as well as BI2536 inhibit growth of vector-expressing and L240F mutant tubulin-expressing cells with similar kinetics and in a dose- and time-dependent manner. Approximately 7%–10% of empty vector-expressing and L240F mutant tubulin-expressing cells were viable at the end of 6 days, and this viability further decreased to 1%–2% at the end of 14 days.
To compare the relative sensitivity of TUBB L240F-expressing cells with that of WT parental cells, clonal cell lines were mixed with uninfected cells and treated with DMSO, rigosertib, or BI2536. Growth curves of mCherry+ cells that stably express TUBB L240F and of vector-expressing cells are shown in Figure 5B. Cells expressing L240F mutant tubulin as well as empty vector-expressing cells that were treated with 400 nM rigosertib had less than 10% viable cells remaining at day 6. When we examined the ratio of mCherry+ cells in this viable fraction, we observed a slightly higher fraction of TUBB L240F-expressing cells in rigosertib-treated cells (Figure 5C). However, we also observed that the fraction of TUBB L240F cells was also higher in BI2536-treated cultures, again confirming that this phenomenon is not unique to rigosertib. Next we examined the levels of β-galactosidase in the remaining cells to quantitate the percentage of senescent cells in the surviving population. These results, shown in Figure 5D, show that 100% of viable cells treated with 200–400 nM rigosertib or 10–20 nM BI2536 were senescent (Figure 5D). This is consistent with published observations that BI2536 is a potent inducer of senescence in certain cell types (Driscoll et al., 2014). As seen with K562 cells, in spite of prolonged incubation in growth medium in the presence of rigosertib, we were unable to establish cell cultures that can grow in the presence of this compound, suggesting that this residual population of cells were truly senescent and do not represent rigosertib-resistant cells. These results strongly suggest that the small fraction of cells that survive rigosertib treatment score positive in viability assays that do not distinguish between proliferating and senescent cells and might lead to misinterpretation of drug resistance.
DISCUSSION
In an earlier study (Athuluri-Divakar et al., 2016), we provided structural and biochemical evidence to show that rigosertib acts as a RAS mimetic, binds to the RBDs of RAF and PI3K family proteins, and disrupts their ability to bind to RAS. This conclusion was based on biochemical assays that included chemical pull-down of target proteins by rigosertib linked to agarose beads, thermal shift assays, and NMR spectroscopy. MST showed that rigosertib binds to RAF RBDs with high affinity, and we were able to solve the structure of the rigosertib-RAF-RBD complex using high-resolution NMR spectroscopy. We also demonstrated that this compound inhibits RAS-mediated activation of the MAPK and AKT pathways in in vitro as well as animal models of RAS-induced tumors (Athuluri-Divakar et al., 2016).
Following publication of our study, Jost et al. (2017) reported that high concentrations of rigosertib (>20 μM) exhibited microtubule-depolymerizing activity and that exogenous expression of a mutant form of tubulin causes tumors cells to become resistant to the effects of rigosertib. Although they did not examine physical binding of rigosertib to tubulins or RBDs of RAS effectors, they provided a crystal structure of a molecule in complex with β-tubulin. Rigosertib preparations purchased from a commercial vendor were used for these studies. Because commercial preparations of drug candidates are of non-GMP quality and often contaminated with intermediates that are used for synthesis of the drug and/or degradation products of the final drug candidate, we examined the purity of rigosertib purchased from Selleckchem. Our studies revealed that the preparation examined by us contained approximately 5% impurities and that the major impurity seen is ON01500, an intermediate in synthesis of rigosertib. ON01500 is known to be a potent tubulin-depolymerizing agent and, hence, could be the agent responsible for the tubulin depolymerization seen by Jost et al. (2017). We compared the tubulin-depolymerization activities of pharmaceutical-grade rigosertib (O-RGS) and commercial-grade rigosertib (S-RGS) and ON01500, the intermediate found in S-RGS preparations. Our results show that ON01500 induces complete depolymerization of tubulin at a concentration of 1 μM and that S-RGS induces depolymerization of tubulin at concentrations of 25 μM and above. Because our previous analysis showed that approximately 5% of S-RGS is contaminated with ON01500, the depolymerizing activity seen above 25 μM S-RGS could be entirely attributed to contaminating ON01500. In the case of O-RGS, we did observe a small delay in tubulin depolymerization at concentrations of 50–100 μM, which could also be attributed to a small (0.1%) contamination of ON01500. Jost et al. (2017) Our conclusion that rigosertib does not bind to β-tubulin is further supported by our binding studies using MST (Wienken et al., 2010), where we measured binding of ON01500, rigosertib, and vincristine, a known tubulin-depolymerizing agent. These studies clearly demonstrate that, although ON01500 and vincristine bind to highly purified preparations of β-tubulin, rigosertib fails to do so.
To determine the crystal structure of the rigosertib-tubulin complex, Jost et al. (2017) used a concentration of 2 mM S-RGS to soak the crystals of β-tubulin. Based on our purity analysis of S-RGS, a 2 mM concentration of S-RGS is expected to contain 100 μM of ON01500 as an impurity, which could have contributed to the structure provided by Jost et al. (2017). ON01500 and rigosertib have minor structural differences, and rigosertib is synthesized from ON01500 by converting the amino group of ON01500 into a glycyl moiety. When we examined the electron density for the unique carboxy group of rigosertib in the structure described by Jost et al. (2017), we found that the density is very weak and that some of the proposed hydrogen bonding interactions are less than optimal. In our opinion, the weak electron density for the carboxyl group in the reported structure can be interpreted as ON01500 and a water molecule, particularly in light of our data showing the presence of ON1500 in commercially sourced rigosertib and the capacity of ON01500 to bind and depolymerize tubulins.
Jost et al. (2017) also reported that expression of the L240F β-tubulin mutant provides resistance to rigosertib, indicating that tubulin binding is critical for its cytotoxic activity. For these experiments, Jost et al. (2017) prepared lentiviral constructs that encoded an empty vector, WT TUBB, or TUBB L240F, which were individually transduced into tumor cells. These cell lines, which express the mCherry marker, were combined at a 1:1 ratio with their respective parental lines and treated with rigosertib or DMSO, and the fraction of TUBB-expressing cells was measured up to 7 days after treatment as mCherry+ cells by flow cytometry. To assess whether expression of TUBB L240F confers resistance to rigosertib, we obtained lentiviral vectors that encode WT tubulin or the L240F β-tubulin mutant from Dr. Weissman’s laboratory and developed several stably transfected clonal K562 and A549 cell lines that express these tubulin proteins. When exponentially growing A549 and K562 clones were treated with increasing concentrations of vehicle or rigosertib, both cell lines that expressed WT or mutant L240F β-tubulin underwent apoptosis with similar kinetics, suggesting that stable expression of L240F β-tubulin does not confer resistance to rigosertib.
In their study, Jost et al. (2017) seeded mutant tubulin-expressing and WT cells at a 1:1 ratio in the same well and measured the ratio of the two populations after treatment with rigosertib or DMSO by flow cytometry, making use of the fact that the tubulin construct was marked with mCherry. An elevated ratio of mCherry-expressing cells after rigosertib treatment compared with DMSO was interpreted to indicate that expression of L240F mutant tubulin confers resistance. To determine the reason for the discrepancy between our studies, we repeated the studies as described by Jost et al. (2017) using the K562 cell line, which was infected with an empty vector or the TUBB L240F expression vector. Seventy-two hours post-infection, the cells were mixed with uninfected cells and treated with DMSO, rigosertib, or BI2536, a pan-PLK inhibitor, as a control. The results of this study showed that WT cells and those expressing TUBB L240F underwent growth inhibition and apoptosis in the presence of rigosertib and BI2536 with similar kinetics, suggesting that expression of mutant TUBB had no effect on the growth-inhibitory activities of rigosertib or BI2536. Cell cultures treated with rigosertib at concentrations of 150 or 200 nM had less than 10% viable cells remaining at day 4 and less than 1% at day 14, consistent with data published previously by us and others (Reddy et al., 2011; Okabe et al., 2015; NCI Developmental Therapeutics Program [https://dtp.cancer.gov/dtpstandard/cancerscreeningdata/index.jsp]). When we examined the ratio of mCherry+ cells in the residual cell fraction at day 4, we observed a slightly higher ratio of mCherry+ cells in TUBB L240F-expressing cell population. Further analysis demonstrated that the majority of the surviving cells exhibited increased β-galactosidase levels, as demonstrated by traditional staining as well as flow cytometric analysis using CellEvent senescence green, confirming that the remaining cells underwent senescence. In spite of prolonged incubation in growth medium, we were unable to establish cultures that could grow in the presence of rigosertib at concentrations of 200 nM, suggesting that this residual population of cells was truly senescent and did not represent rigosertib-resistant cells. Next we repeated these studies with K562 and A549 cell lines stably infected with TUBB L240F expression vectors and selected for mCherry expression. When these cells were mixed with their parental cell lines and examined for their sensitivity to rigosertib or BI2436, we again observed that TUBB L240F expression did not confer any growth advantage. At the end of 4 days of incubation with rigosertib, both parental cells and their TUBB L240F-expressing counterparts underwent apoptosis with similar kinetics, and by this time, less than 10% of cells in each population were viable. When we examined the ratio of mCherry+ cells in this viable fraction, we observed a slightly higher percentage of TUBB L240F-expressing cells among rigosertib-treated cells. However, we also observed that the population of TUBB L240F cells was higher in BI2536-treated cells, suggesting that this phenomenon is not unique to rigosertib. More importantly, when we examined β-galactosidase levels in the surviving cells to quantify the percentage of senescent cells, we found that significant percentages of the remaining viable cells treated with 200–400 nM rigosertib or 10–20 nM BI2536 were senescent (Figures 4 and 5).
Because definitive proof of drug resistance is to establish permanent cell lines that grow in 4- to 5-fold higher concentrations of the drug, we made repeated attempts to establish permanent cell lines that express L240F-TUBB and proliferate in the presence of 3- to 4-fold higher concentrations of rigosertib that are otherwise lethal to WT cells. Despite several attempts, we have not been able to establish any cell lines that grow at a concentration that is otherwise lethal to WT cells. Based on these studies, we conclude that the L240F mutant form of β-tubulin does not confer resistance to rigosertib.
Rigosertib is a small-molecule therapeutic agent currently in phase III clinical trials for treatment of myelodysplastic syndrome and cancers that are commonly RAS mutated. This drug has been administered to more than 1,000 cancer patients, and none experienced the typical side effects induced by tubulin-depolymerizing agents, such as peripheral neuropathy, bone marrow suppression, hair loss, and gastrointestinal problems (Lu et al., 2012), further suggesting that rigosertib has a different mechanism of action compared with any of the known tubulin-binding agents.
Based on this analysis, we conclude that some of the results obtained by Jost et al. (2017) are due to contamination of commercial-grade rigosertib with ON01500, an intermediate in synthesis of rigosertib and a potent tubulin-polymerizing agent. In addition, the assays used to determine resistance relied on short-term culture assays, which did not take into account that many targeted therapies induce senescence of tumor cells, which were scored as a proliferating cell population in flow cytometry analyses.
STAR★METHODS
RESOURCE AVAILABILITY
Contact for Reagent and Resource Sharing
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, E. Premkumar Reddy (ep.reddy@mssm.edu).
Materials Availability
Request for materials generated in this study should be directed to the Lead Contact and will be made available upon execution of a Material Transfer Agreement.
Data Code and Availability
This study did not generate any unique datasets or code.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Cell Lines
HEK293 and A549 cells were obtained from the American Type Culture Selection (ATCC) cultured in Dulbecco’s Modified Eagle Medium (DMEM) (Life Technologies) supplemented with 10% FBS and penicillin-streptomycin. Cell lines were authenticated by ATCC prior to purchase. K562 cells were obtained from Jonathan Weissman and grown in RPMI (Life Technologies) supplemented with 10% FBS and penicillin-streptomycin. All cells were at 37°C grown under humidified conditions and 5% CO2. Cell lines were not independently authenticated.
METHOD DETAILS
Additional details can be found in the Key Resources Table and on the online STAR Methods website.
KEY RESOURCES TABLE.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and Virus Strains | ||
| pHR-SFFV-TUBB L240F-IRES-mCherry | Jost et al., 2017 | N/A |
| pHR-SFFV-TUBBL-IRES-mCherry | Jost et al., 2017 | N/A |
| pHR-SFFV-IRES-mCherry | Jost et al., 2017 | N/A |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Rigosertib (pharmaceutical grade) | Onconova Therapeutics, Inc | N/A |
| Rigosertib (chemical grade) | Selleck Chemicals | Cat# S1362 |
| ON01500 | Onconova Therapeutics, Inc | N/A |
| vincristine | Sigma-Aldrich | Cat# V8879 |
| BI2536 | Selleck Chemicals | Cat# S1109 |
| Prionex | Sigma-Aldrich | Cat# G0411-100ML |
| eBioscience Fixable Viability Dye eFluor 450 | ThermoFisher Scientific | Cat# 65-0863-14 |
| MAP-rich tubulin | Cytoskeleton, Inc | Cat# ML116 |
| Tubulin | Cytoskeleton, Inc | Cat# T240 |
| General Tubulin Buffer | Cytoskeleton, Inc | Cat# BST-06 |
| Tubulin Cushion Buffer | Cytoskeleton, Inc | Cat# BST05-001 |
| Tubulin Polymerization Buffer | Cytoskeleton, Inc | Cat# BP01 |
| Critical Commercial Assays | ||
| Tubulin Polymerization Assay Kit | Cytoskeleton, Inc | Cat# BK011P |
| Monolith NT Protein RED-NHS Labeling Kit | NanoTemper | Cat# MO-0001 |
| CellEvent Senescence Green | ThermoFisher Scientific | Cat# C10841 |
| CellTiter Blue | Promega | Cat# G8080 |
| Deposited Data | ||
| Crystal structure of tubulin rigosertib | Jost et al., 2017 | PDB:5OV7 |
| Experimental Models: Cell Lines | ||
| K562-TUBB L240F-mCherry | This paper | N/A |
| K562-mCherry | This paper | N/A |
| A549-TUBB L240F-mCherry | This paper | N/A |
| A549-mCherry | This paper | N/A |
| A549 | American Type Culture Collection | Cat# CCL-185 |
| K562 | Jost et al., 2017 | N/A |
| HEK293T | American Type Culture Collection | Cat# CRL-3216 |
| Recombinant DNA | ||
| pHR-SFFV-TUBB L240F-IRES-mCherry | Jost et al., 2017 | N/A |
| pHR-SFFV-IRES-mCherry | Jost et al., 2017 | N/A |
| pVSV-G | Stewart et al., 2003 | Addgene #138479 |
| pδVPR | Stewart et al., 2003 | Addgene #8455 |
| Software and Algorithms | ||
| Prism v8.0 | GraphPad Software, LLC | N/A |
| FlowJo v10.6.1 | Treestar | N/A |
| NCI Developmental Therapeutics Program | The National Cancer Institute | https://dtp.cancer.gov/dtpstandard/cancerscreeningdata/index.jsp |
| Other | ||
| Methocult base methylcellulose medium | StemCell Technologies | Cat# H4100 |
| 96-well imaging plates | Fisher Scientific | Cat# 08-772-225 |
| 6-well Half Area Black Flat Bottom Polystyrene NBS Microplate | Corning-Costar | Cat# 3686 |
| Synergy H1 Multi-mode Hybrid Reader | BioTek | N/A |
| Monolith NT.115 | NanoTemper | N/A |
| Monolith NT.115 Standard Capillaries | NanoTemper | Cat# MO-K002 |
Chemicals
GMP-grade rigosertib (RGS) and ON01500 were obtained from Onconova Therapeutics, Inc. Chemical grade RGS and BI2536 were purchased from Selleck Chemicals. Vincristine was purchased from Sigma Aldrich.
LC/MS/MS
The two compounds were analyzed on Agilent 6410 triple quadrupole mass spectrometer. Samples were dissolved in acetonitrile and eluted in 60% ammonium acetate and 40% acetonitrile on C18 column at a flow rate of 0.2 mL/min. The analytes were monitored by tandem mass spectrometry with electrospray positive ionization.
Plasmids and Generation of Lentiviruses
Lentiviral supernatants and plasmids encoding wild-type and L240F mutant TUBB were obtained from Jonathan Weissman (Jost et al., 2017) and co-transfected with packaging plasmids (Singleton and Wood, 2016) using Lipofectamine 2000 (Thermo Fisher Scientific) into HEK293T cells to generate recombinant lentiviruses. Viral supernatant was harvested 48 and 72 hr post-transfection and filtered prior to further use.
Generation of Tubulin-expressing cells
Tubulin-expressing and vector control cell lines were generated by spinoculation. Briefly, 1×106 cells were infected with 100–150μl of virus in the presence of 7.5mg/ml polybrene and centrifuged at 2,200 rpm at 37°C for 1 hr. Media was changed the following day and the cells grown for an additional 48 hr prior to use. Stable transfectants were infected as described above, grown in the presence of 2μg/ml puromycin and clonal cell lines further selected using limiting dilution. The percentage of mCherry+ cells was determined by flow cytometric analysis as described below. Cell lines with > 95% mCherry+ cells were chosen for further analysis.
Growth and Viability Assays
To determine the growth kinetics of tubulin-expressing K562 and A549 cells, clonal cell lines were seeded at a density of 1×105/ml (K562) or 1×106 cells in a 100mm dish (A549). Cell number and viability were determined on the indicated days by trypan blue exclusion. For 96 hr dose response assays, exponentially growing cells were seeded at a density of 2.5×103/well of a 96-well plate and treated the same day (K562) or the following day (A549) with the indicated concentrations of GMP-grade RGS. Viability was determined 96 hours post-treatment using CellTiter Blue (Promega) according to the instructions of the manufacturer.
To assess proliferation in 3-demensional assays, 1×105 cells were seeded in 35mm dishes in duplicate in H4100 base methocult (StemCell Technologies, Inc.), supplemented with 10% FBS and the recommended volume of IMDM in the presence increasing concentrations of rigosertib. Plates were incubated for 6 days at 37°C under humidified conditions and 5% CO2. Images were acquired using an Olympus microscope using a 20X objective and manufacturer supplied software.
Tubulin Polymerization Assays
25μg of MAP-rich tubulin (Cytoskeleton, Inc.; #ML116) in general tubulin buffer (80mM PIPES, pH 6.9/ 2mM MgCl2/ 0.5mM EGTA/10μM fluorescence reporter) (Cytoskeleton, Inc.; #BP01) was combined with 1mM GTP, 15% tubulin glycerol cushion buffer (Cytoskeleton, Inc.; # BPST05-001) and the indicated concentrations of rigosertib (obtained from either Selleckchem [chemical grade] or Onconova Therapeutics, Inc. [pharmaceutical grade]), ON01500 (Reddy et al., 2011), and vincristine (Sigma-Aldrich; #V8879). All compounds were dissolved in DMSO and used as 25X stock solutions. The reactions were incubated at 37°C in a Synergy H1 Multi-mode Hybrid Reader (BioTek) and fluorescence measured every 30 s over the indicated period of time. Fluorescence values were normalized by subtracting the points obtained at the start of polymerization for each reaction from all subsequent data points.
Microscale Thermophoresis
Tubulin (Cytoskeleton, Inc.; #T240) was labeled for microscale thermophoresis (MST) using the Monolith NT Protein RED-NHS Labeling Kit (NanoTemper Technologies, München, Germany) according to the instructions of the manufacturer. Briefly, tubulin at a concentration of 20 μM was incubated with 2X dye at a ratio of 1:1 in labeling buffer (manufacturer supplied) in the dark at room temperature for 30 minutes. Free dye was removed using a manufacturer supplied gel filtration column and the protein eluted in 0.5ml of general tubulin buffer (80mM PIPES, pH 6.9/ 2mM MgCl2/ 0.5mM EGTA). To determine the Kd values of rigosertib, ON01500 and vincristine to tubulin, labeled tubulin was incubated with increasing concentrations of compound in reaction buffer (20mM Tris-HCl pH 7.5/ 150mM NaCl/ 1mM MgCl2/ 2% DMSO/ 0.5% prionex) for 30 minutes in the dark at room temperature. All compounds were used as 100X stock solutions. The samples were then centrifuged at 13,000 rpm for 2 minutes before being loaded into standard capillaries supplied by the manufacturer. Fluorescence values from the binding reactions were determined using the Monolith NT.115 (NanoTemper Technologies, München, Germany). Binding data was analyzed using GraphPad Prism (GraphPad Software, San Diego, CA) to determine Kd values. For these analyses, the fluorescence value from the thermophoresis plots corresponding to the lowest concentration of compound used in the titration was subtracted from every data point prior to normalization. In the case of rigosertib (for which a curve could not be fit), the highest value was set to 100 and the data normalized accordingly.
Flow Cytometry
For analysis of mCherry expression and sorting of viable mCherry+/− cells, single cell suspensions were prepared in PBS supplemented with 2% FBS and 1μg/ml DAPI. Levels of senescence in K562 clonal cell lines were measured using the CellEvent Senescence Green flow cytometry assay kit according to the instructions of the manufacturer (Thermo Fisher Scientific) except that cells were stained with fixable viability dye eFluor 450 (Thermo Fisher Scientific) for 30 min at 4°C prior to fixation according to the manufacturer’s instructions. To analyze β-galactosidase levels in cultures that were a mixture of mCherry+ and wild-type (mCherry−) cells, viable (DAPI-) mCherry+ and mCherry− cells were sort purified prior to fixation in 2% paraformaldehyde, washed extensively with phenol red-free RPMI containing 2% FBS and stored overnight at 4°C prior to staining with CellEvent Senescence Green (Thermo Fisher Scientific). Flow cytometric analysis and sorting was performed at the Flow Cytometry shared Resource Facility at the Icahn School of Medicine at Mount Sinai. Data for analytical purposes were acquired using an LSRFortessa X-20 or FACSCantoII (BD Biosciences). Sorted cell populations were obtained using an Arial II (BD Biosciences) high-speed sorter. All data were analyzed using FlowJo v10.6.1 (Treestar) software.
Senescence Studies
β-galactosidase levels in K562 cell lines were determined as described in the flow cytometry section. To analyze levels of β-galactosidase in A549 cells, cells were washed with PBS and fixed in PBS containing 2% formaldehyde/0.2%gluteraldehyde for 5 minutes at room temperature. The cells were then washed twice with PBS and stained with 0.1mg/ml X-gal/40mM citric acid sodium phosphate buffer (0.1M citric acid monohydrate/0.2M Na2HPO4), pH 6.0/ 5mM potassium ferrocyanide/ 5mM ferricyanide/ 150mM NaCl/ 2mM MgCl2 overnight at 37°C. Images were acquired using an Olympus microscope using a 20X objective and manufacturer supplied software.
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistical analysis was performed using Prism 8.0 (GraphPad Software, LLC). Data are represented as mean ± SEM.
Highlights.
Rigosertib does not bind directly to tubulin
Rigosertib dos not inhibit tubulin polymerization
Expression of TUBB L240F does not confer resistance to rigosertib
A small percentage of rigosertib- and BI2536-treated TUBB L240F cells undergo senescence
ACKNOWLEDGMENTS
We thank Dr. Jonathan Weissman for providing the lentiviral constructs encoding WT and L240F β-tubulin as well as lentiviruses encoding the same. This work was supported by grants from Onconova Therapeutics Inc. (Newtown, PA), the U.S. Army Medical Research and Materiel Command (LC160287), and the National Institutes of Health (NIH) (5R21CA227963-02) (to E.P.R.). Use of the flow cytometry shared resource facility was supported by a NIH Cancer Center support grant (P30CA196521 to the Tisch Cancer Institute).
Footnotes
DECLARATION OF INTERESTS
E.P.R. is an equity holder, board member, and paid consultant of Onconova Therapeutics. S.J.B. is a paid consultant of Onconova Therapeutics. M.V.R.R. and S.C.C. are stockholders and paid consultants of Onconova Therapeutics, Inc. M.V.R.R. and E.P.R. are named inventors on pending and/or issued patents filed by Temple University.
REFERENCES
- Agoni L, Basu I, Gupta S, Alfieri A, Gambino A, Goldberg GL, Reddy EP, and Guha C (2014). Rigosertib is a more effective radiosensitizer than cisplatin in concurrent chemoradiation treatment of cervical carcinoma, in vitro and in vivo. Int. J. Radiat. Oncol. Biol. Phys. 88, 1180–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athuluri-Divakar SK, Vasquez-Del Carpio R, Dutta K, Baker SJ, Cosenza SC, Basu I, Gupta YK, Reddy MV, Ueno L, Hart JR, et al. (2016). A Small Molecule RAS-Mimetic Disrupts RAS Association with Effector Proteins to Block Signaling. Cell 165, 643–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll DL, Chakravarty A, Bowman D, Shinde V, Lasky K, Shi J, Vos T, Stringer B, Amidon B, D’Amore N, and Hyer ML (2014). Plk1 inhibition causes post-mitotic DNA damage and senescence in a range of human tumor cell lines. PLoS ONE 9, e111060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimeno A, Chan A, Cusatis G, Zhang X, Wheelhouse J, Solomon A, Chan F, Zhao M, Cosenza SC, Ramana Reddy MV, et al. (2009). Evaluation of the novel mitotic modulator ON 01910.Na in pancreatic cancer and preclinical development of an ex vivo predictive assay. Oncogene 28, 610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost M, Chen Y, Gilbert LA, Horlbeck MA, Krenning L, Menchon G, Rai A, Cho MY, Stern JJ, Prota AE, et al. (2017). Combined CRISPRi/a-Based Chemical Genetic Screens Reveal that Rigosertib Is a Microtubule-Destabilizing Agent. Mol. Cell 68, 210–223.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Chen J, Xiao M, Li W, and Miller DD (2012). An overview of tubulin inhibitors that interact with the colchicine binding site. Pharm. Res. 29, 2943–2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe S, Tauchi T, Tanaka Y, Sakuta J, and Ohyashiki K (2015). Efficacy of the polo-like kinase inhibitor rigosertib, alone or in combination with Abelson tyrosine kinase inhibitors, against break point cluster region-c-Abelson-positive leukemia cells. Oncotarget 6, 20231–20240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel HH, Maniar M, Ren C, and Dave RH (2018). Determination of Degradation Kinetics and Effect of Anion Exchange Resin on Dissolution of Novel Anticancer Drug Rigosertib in Acidic Conditions. AAPS PharmSciTech 19, 93–100. [DOI] [PubMed] [Google Scholar]
- Reddy MV, Venkatapuram P, Mallireddigari MR, Pallela VR, Cosenza SC, Robell KA, Akula B, Hoffman BS, and Reddy EP (2011). Discovery of a clinical stage multi-kinase inhibitor sodium (E)-2–2-methoxy-5-[(2′,4′,6′-trimethoxystyrylsulfonyl)methyl]phenylaminoacetate (ON 01910.Na): synthesis, structure-activity relationship, and biological activity. J. Med. Chem. 54, 6254–6276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman LR, Greenberg P, Raza A, Olnes MJ, Holland JF, Reddy P, Maniar M, and Wilhelm F (2015). Clinical activity and safety of the dual pathway inhibitor rigosertib for higher risk myelodysplastic syndromes following DNA methyltransferase inhibitor therapy. Hematol. Oncol. 33, 57–66. [DOI] [PubMed] [Google Scholar]
- Singleton KR, and Wood KC (2016). Narrowing the focus: a toolkit to systematically connect oncogenic signaling pathways with cancer phenotypes. Genes Cancer 7, 218–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart SA, Dykxhoorn DM, Palliser D, Mizuno H, Yu EY, An DS, Sabatini DM, Chen IS, Hahn WC, Sharp PA, et al. (2003). Lentivirus-delivered stable gene silencing by RNAi in primary cells. RNA 9, 493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wienken CJ, Baaske P, Rothbauer U, Braun D, and Duhr S (2010). Protein-binding assays in biological liquids using microscale thermophoresis. Nat. Commun. 1, 100. [DOI] [PubMed] [Google Scholar]


