Figure 1. Purity and Tubulin-Binding Activities of Pharmaceutical-Grade and Commercial-Grade Rigosertib.
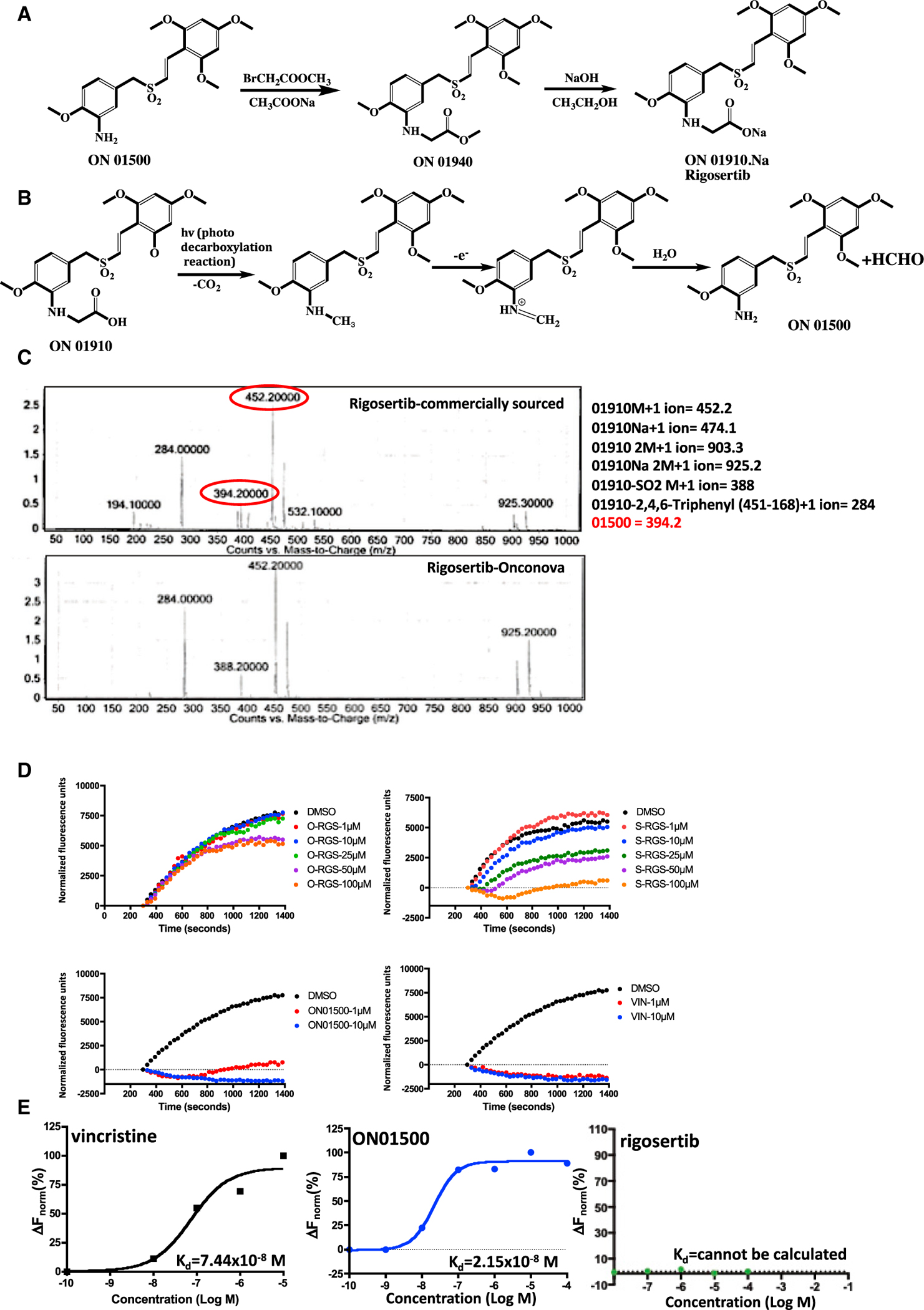
(A) Synthetic scheme used for preparation of rigosertib (Reddy et al., 2011).
(B) Photodegradation of rigosertib to ON01500.
(C) LC-MS/MS analysis of rigosertib obtained from Onconova Therapeutics and Selleckchem.
(D) Polymerization of tubulin in the presence of rigosertib from Onconova Therapeutics (O-RGS) and Selleckchem (S-RGS), ON01500 (Onconova Therapeutics), and vincristine (VIN). 25 μg of MAP-rich tubulin in general tubulin buffer was mixed with 1 mM guanosine triphosphate (GTP) and fluorescence reporter in the presence of vehicle (DMSO) or increasing concentrations of the indicated compound. Tubulin polymerization as a function of fluorescence was recorded over the indicated time at 37°C.
(E) Microscale thermophoretic analysis of ON01500 and VIN with purified tubulin. Tubulin was labeled using the Monolith NT protein labeling kit RED-NHS according to the instructions of the manufacturer. Labeled protein was incubated with increasing concentrations of rigosertib, ON01500, or VIN for 30 min and subjected to MST.
