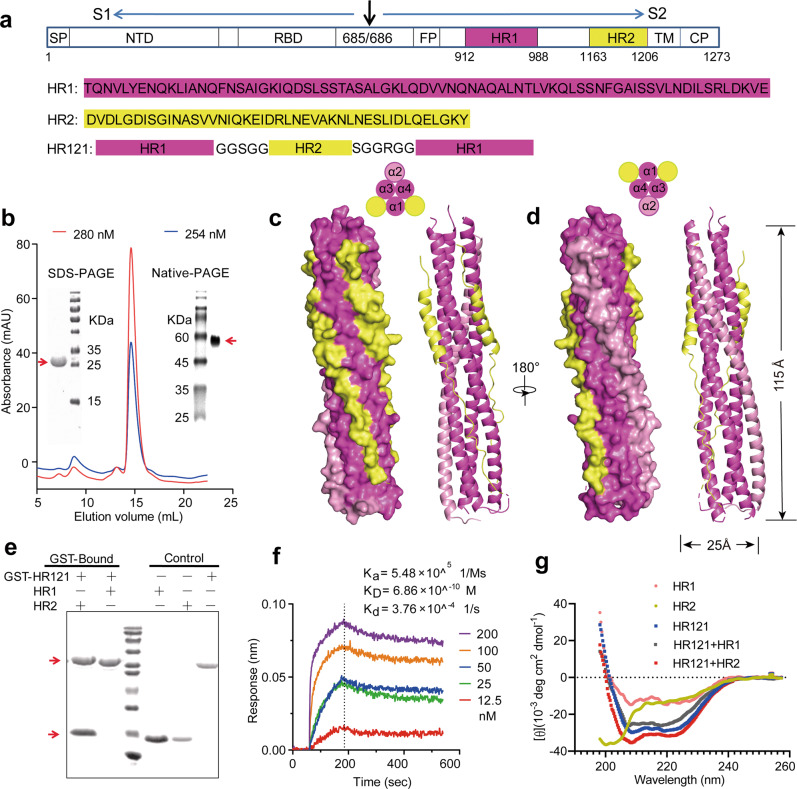
Fig. 1. Design and characterization of the recombinant protein HR121.
a Amino acid sequence of HR121. HR1 and HR2 peptide sequences were derived from the HR1 and HR2 domains, respectively, in the S protein of the prototype SARS-CoV-2 Wuhan-Hu-1 strain. HR121 monomer consists of HR1–linker 1–HR2–linker 2–HR1 segments (linker 1: GGSGG; linker 2: SGGRGG). Signal peptide (SP), N-terminal domain (NTD), and receptor-binding domain (RBD) in S1 subunit, and fusion peptide (FP), HR1 (aa 912–988), HR2 (aa 1163–1206), transmembrane domain (TM) and cytoplasmic domain (CP) in S2 subunit, were marked in the schematic diagram. b Purification of HR121. HR121 protein was purified using a Superdex 200 Increase 10/300 GL column; the profiles of the representative elution, SDS-PAGE and Native-PAGE were presented (the red arrows point the band of HR121 in SDS-PAGE and Native-PAGE gels); mAU in y-axis means milli-absorbance units. c, d Surface and cartoon representation of the atomic model of HR121 dimer. HR121 dimer consists of four HR1 (α1–α4) and two HR2. Front view (c) displayed the inner HR1 trimer (α1, α3 and α4) surrounded by two HR2s and one HR1 (α2), and back view (d) showed the two dominant-negative interfaces (between α2 and α3, α2 and α4) formed in the outer HR1 trimer (α2, α3 and α4). e GST pull-down assay showed that HR121 selectively bound to HR2, but not to HR1 (the red arrow at upper position points the band of GST-HR121, while red arrow at lower position points the band of HR2 or HR1). f Surface plasmon resonance recorded the profile of a real-time affinity of HR2 to HR121. g CD spectroscopic analysis showed that HR121 could interact with HR2 to form a more stable α-helix.