Abstract
Background:
Molecular testing for JAK2 mutations is part of the standard diagnostic workup for patients with suspected polycythemia vera. We sought to characterize evolving practice patterns in the investigation of erythrocytosis and the prevalence of secondary causes, including use of medications such as sodium–glucose cotransporter-2 (SGLT2) inhibitors, among patients who underwent molecular testing.
Methods:
We reviewed charts of all consecutive patients investigated for erythrocytosis (hemoglobin > 160 g/L for women, > 165 g/L for men) with JAK2 testing between 2015 and 2021 at London Health Sciences Centre, a tertiary referral centre in Ontario, Canada, to assess changes in rates of JAK2 mutation positivity, average hemoglobin levels and the prevalence of secondary causes of erythrocytosis.
Results:
A total of 891 patients with erythrocytosis underwent JAK2 mutation testing with an increase in number of tests (particularly from 2017 to 2018), a decrease in the rate of JAK2 positivity and similar average hemoglobin levels over the study period. We observed a high proportion of patients with secondary causes of erythrocytosis, ranging from 59% to 74% over the study period, including medications associated with erythrocytosis, namely testosterone (6%–11%) and SGLT2 inhibitors (2%–19%). Stopping SGLT2 inhibitors was associated with a significant decrease in hemoglobin levels (mean −14.7 g/L, 95% confidence interval −18.9 to −10.5 g/L) compared with continuation.
Interpretation:
Use of SGLT2 inhibitors may be a common and underrecognized secondary cause of elevated hemoglobin levels in patients investigated for erythrocytosis. Our findings underscore the importance of a detailed medical history to support judicious use of molecular testing, in adherence with the current guideline on the investigation of erythrocytosis.
Erythrocytosis is defined as a concentration of red blood cells higher than age- and sex-specific reference ranges, most commonly measured as an increase in the hemoglobin level or hematocrit. Since the identification of the JAK2 V617F mutation in patients with polycythemia vera in 2005,1 molecular testing of the JAK2 gene has become part of the standard diagnostic workup for patients presenting with erythrocytosis. The increasing availability of molecular diagnostics and a decrease in hemoglobin thresholds (> 160 g/L for women, > 165 g/L for men) required to diagnose polycythemia vera in the revised 2016 World Health Organization (WHO) classification2 are 2 potential factors driving increased use of molecular testing in patients with erythrocytosis.3
Secondary causes of erythrocytosis — such as smoking, hypoxic lung diseases and medications — are more common than polycythemia vera, and current literature supports first excluding these causes by taking a thorough medical history and by focused investigations, including serum erythropoietin levels, before performing molecular testing.4 Despite these recommendations, in practice, this work-up is often performed concurrently with molecular testing, which can carry substantial health care costs.
The primary objective of this study was to characterize practice patterns in the investigation of erythrocytosis at our centre, focusing on the use of molecular testing for JAK2 mutations in a real-world cohort of patients referred for elevated hemoglobin levels. We hypothesized that an increase in molecular testing from 2015 to 2021 might have resulted from the lower hemoglobin thresholds in the revised 2016 WHO diagnostic criteria for polycythemia vera or from improved access to JAK2 testing. A secondary objective was to assess the prevalence of secondary causes of erythrocytosis in patients who underwent JAK2 testing, including use of testosterone and sodium–glucose cotransporter-2 (SGLT2) inhibitors (both established but potentially underrecognized secondary causes of erythrocytosis).4–6
Methods
Setting and study design
We conducted this study at London Health Sciences Centre, a tertiary referral centre that serves a population of about 2 million people in southwestern Ontario, Canada.
We retrospectively reviewed charts from all consecutive patients aged 18 years or older who were investigated at our centre for erythrocytosis (hemoglobin > 160 g/L for women, > 165 g/L for men) with JAK2 mutation testing between Aug. 1, 2015, and May 20, 2021. We selected the study period based on the availability of data for JAK2 mutation testing. All tests were conducted in the Molecular Diagnostics Division at London Health Sciences Centre and data were available for all patients who underwent testing during the specified time period; therefore, we were able to capture all tests in our study.
Chart review was performed in triplicate, with a minimum of 3 study authors reviewing each chart, including at least 1 hematology specialist (B.C.-Y., A.L.-L., I.C.-Y., C.H.). We completed a detailed data collection form for each case, including patient demographics, comorbidities and medication history.
Molecular analysis
Between 2015 and 2017, JAK2 mutation testing was performed by quantitative polymerase chain reaction (qPCR) using the Roche 480 LightCycler (La Roche AG). Between 2018 and 2020, this testing was done by single nucleotide polymorphism (SNP) allelotyping using the Agena MassARRAY system (Agena Biosciences) or next-generation sequencing (NGS) using the Oncomine Myeloid Research Assay (ThermoFisher Scientific). Specifically, qPCR and SNP allelotyping assays detected the JAK2 V617F mutation; the NGS assay detected any JAK2 mutations, including V617F and mutations in exons 12–15.
Data collection
We performed a chart review to extract laboratory and clinical data, including hemoglobin levels and information on medical comorbidities and medications, with a focus on known secondary causes of erythrocytosis.
Statistical analysis
We performed statistical analysis in R (version 4.1.0) using the Fisher exact test and the Mann–Whitney U test. We tested differences in hemoglobin by the Mann–Whitney U test, but used the parametric t-distribution approach to construct 95% confidence intervals (CIs). We also conducted an analysis of patients who underwent molecular testing for isolated thrombocytosis for direct comparison of key factors such as exposure to SGLT2 inhibitors and testosterone.
Ethics approval
This study was approved by the Research Ethics Boards at Western University (no. 118139) and the Lawson Research Institute (no. 10750).
Results
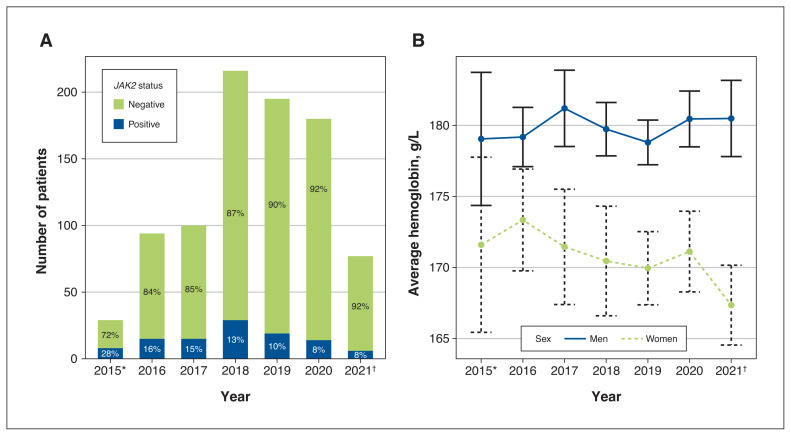
A total of 891 consecutive patients with erythrocytosis underwent JAK2 mutation testing at our institution from Aug. 1, 2015, to May 20, 2021. Patient characteristics are shown in Table 1. The number of patients who underwent JAK2 mutation testing increased over the study period, with a decline in the positive detection rate; 8 (27.6%) of 29 patients in 2015, 15 (16.0%) of 94 patients in 2016, 15 (15.0%) of 100 patients in 2017, 29 (13.4%) of 216 patients in 2018, 19 (9.7%) of 195 patients in 2019, 14 (7.8%) of 180 patients in 2020 and 6 (7.8%) of 77 patients in 2021 tested positive (Figure 1A). The average hemoglobin levels in patients with erythrocytosis who underwent testing remained similar across the study period (average 167–173 g/L for women, 179–181 g/L for men; Figure 1B).
Table 1:
Patient characteristics at baseline
| Characteristic | Men n = 644 |
Women n = 247 |
Total n = 891 |
|---|---|---|---|
| Age,* yr, mean ± SD | 58 ± 14 | 61 ± 14 | 59 ± 14 |
| Height, cm, mean ± SD | 176 ± 8 | 161 ± 7 | 172 ± 10 |
| Weight, kg, mean ± SD | 97 ± 21 | 80 ± 20 | 92 ± 22 |
| BMI, mean ± SD | 31 ± 6 | 31 ± 8 | 31 ± 7 |
| Hemoglobin, g/L, mean ± SD | 180 ± 11 | 171 ± 10 | 177 ± 11 |
| Hematocrit, L/L, mean ± SD | 0.54 ± 0.04 | 0.52 ± 0.04 | 0.53 ± 0.04 |
| Leukocytes, × 109 cells/L, mean ± SD | 8.9 ± 3.6 | 9.6 ± 4.0 | 9.1 ± 3.7 |
| Platelets, × 109 cells/L, mean ± SD | 250 ± 144 | 326 ± 217 | 271 ± 171 |
Note: BMI = body mass index, SD = standard deviation.
Age was determined at the time of molecular testing.
Figure 1:
(A) Number of patients and JAK2 positivity rates between 2015 and 2021. (B) Average hemoglobin levels for men and women tested for JAK2 mutations each year. *2015 includes only patients tested from Aug. 1 to Dec. 31, 2015. †2021 includes only patients tested from Jan. 1 to May 20, 2021.
In our cohort of patients who underwent molecular testing, the proportion with documented secondary causes of erythrocytosis was consistently high, ranging from 59% to 75% between 2015 and 2021. Secondary causes included smoking, chronic obstructive pulmonary disease, obstructive sleep apnea, other hypoxic lung disease, erythropoietin-secreting tumour or medications (Table 2). We observed an increase in the use of SGLT2 inhibitors among patients who underwent JAK2 testing, with 0 (0.0%) of 29 patients in 2015, 2 (2.1%) of 94 patients in 2016, 13 (13.0%) of 100 patients in 2017, 17 (7.9%) of 216 patients in 2018, 18 (9.2%) of 195 patients in 2019, 32 (17.8%) of 180 patients in 2020 and 15 (19.5%) of 77 patients in 2021 using SGLT2 inhibitors. The proportion of patients on testosterone was relatively constant at 2 (6.9%) of 29 patients in 2015, 10 (10.6%) of 94 patients in 2016, 7 (7.0%) of 100 patients in 2017, 22 (10.2%) of 216 patients in 2018, 15 (7.7%) of 195 patients in 2019, 14 (7.8%) of 180 patients in 2020 and 5 (6.5%) of 77 patients in 2021. Rates of SGLT2 inhibitor and testosterone use were significantly higher among JAK2-negative patients than among JAK2-positive patients (p < 0.001 for SGLT2 inhibitors; p = 0.001 for testosterone) (Table 2).
Table 2:
Secondary causes of erythrocytosis, stratified by presence of JAK2 mutation
| Cause* | No. of patients | p value | |
|---|---|---|---|
| Positive for JAK2 mutation n = 106 |
Negative for JAK2 mutation n = 785 |
||
| Smoking | 25 (23.6) | 319 (40.6) | < 0.001 |
| OSA | 6 (5.7) | 251 (32.0) | < 0.001 |
| COPD | 5 (4.7) | 107 (13.6) | 0.007 |
| Other hypoxic lung diseases | 6 (5.7) | 83 (10.6) | 0.12 |
| SGLT2 inhibitors | 2 (1.9) | 95 (12.1) | < 0.001 |
| Testosterone use | 1 (0.9) | 73 (9.3) | 0.001 |
| EPO-secreting tumour | 0 (0.0) | 5 (0.6) | 1.0 |
Note: COPD = chronic obstructive pulmonary disease, EPO = erythropoietin, OSA = obstructive sleep apnea, SGLT2 = sodium–glucose cotransporter-2.
Patients may have more than 1 secondary cause.
To ascertain if this observation was specific to patients with erythrocytosis, we also reviewed data for all patients who underwent molecular testing for isolated thrombocytosis (platelet count > 450 × 109/L) from 2018 to 2021; 5 (1.1%) of 446 patients with isolated thrombocytosis were on SGLT2 inhibitors, compared with 82 (12.3%) of 668 patients with erythrocytosis during the same time period (p < 0.001). Likewise, a significantly lower proportion of patients with thrombocytosis (n = 2 [0.4%]) were on testosterone, compared with patients with erythrocytosis (n = 55 [8.2%], p < 0.001).
Of note, 2 patients on SGLT2 inhibitors were found to have JAK2 V617F mutations and received diagnoses of polycythemia vera. Although their hemoglobin levels (both 170 g/L) were similar at presentation to the average of this cohort, both patients also had thrombocytosis, and one had leukocytosis and associated symptoms suggestive of a myeloproliferative neoplasm, including aquagenic pruritus and unexplained weight loss. To evaluate the impact of SGLT2 inhibitors on erythrocytosis, we reviewed all remaining patients on SGLT2 inhibitors in the cohort (n = 95). Follow-up data were available for 91 patients. Sixteen patients had confounding factors, such as phlebotomy, acute illness or other drug-induced causes (e.g., testosterone), which precluded evaluation of follow-up hemoglobin levels. Of the remaining 75 patients, 15 (20.0%) stopped the SGLT2 inhibitor and 60 (80.0%) continued the medication. The average difference between the hemoglobin level at the time of referral and the maximum hemoglobin level during follow-up was −14.7 (95% CI −18.9 to −10.5) g/L for those who stopped SGLT2 inhibitors and 0.22 (95% CI −1.77 to 2.33) g/L for those who continued these medications (p < 0.001).
Interpretation
In this study, we found changing patterns in the investigation of erythrocytosis in a real-world population, indicating an increase in molecular testing over time, with a decline in the detection rate of JAK2 mutations. Although we initially hypothesized that an increase in JAK2 testing may have resulted from a change in the WHO diagnostic criteria in 2016, the average hemoglobin levels among tested patients remained relatively constant over the study period, suggesting that the main drivers of increased testing were likely related to increased awareness of and accessibility to molecular diagnostics. Indeed, in 2018 the myeloid NGS panel was introduced into practice at our centre, and this increased availability of testing may have been a major factor behind the change in number of tests over time. This is further supported by the high proportion of patients with documented secondary causes of erythrocytosis who underwent molecular testing over the study period.
We also found an increase in the use of SGLT2 inhibitors among patients with erythrocytosis who underwent JAK2 mutation testing. This finding confirms previous clinical observations by our group,3,6 and has since also been reported in another single-centre retrospective study, which identified 30 patients with SGLT2 inhibitor–induced erythrocytosis.7 These drugs are known to cause an increase in hemoglobin levels, an effect noted in initial clinical trials and hypothesized to contribute to their cardioprotective effects.8,9 The mechanisms of SGLT2 inhibitor–induced erythrocytosis remain to be elucidated but have been postulated to include hemoconcentration due to mild diuretic effect, modulation of iron metabolism by suppression of hepcidin10 and to stimulation of renal erythropoietin production.11 In a posthoc analysis of the EMPAREG OUTCOME trial, a mean increase in hemoglobin of 8 g/L was observed among patients who used empagliflozin.9 Literature on more severe cases of erythrocytosis associated with SGLT2 inhibitor use is limited to a small number of case reports;12–14 however, the findings of our study suggest that this may be a more widespread phenomenon and that SGLT2 inhibitors may be a significant, underrecognized cause of drug-induced erythrocytosis. Indeed, at our centre we have noted an increase in referrals for patients with erythrocytosis on SGLT2 inhibitors.6 Sodium–glucose cotransporter-2 inhibitors are a class of medications that are increasingly prescribed based on evidence of improved cardiovascular and renal outcomes;15 indications for their use have expanded beyond type 2 diabetes mellitus to also include chronic kidney disease and heart failure. 16 Considering this growing use, health care providers should be aware of the potential for SGLT2 inhibitors to cause elevations in hemoglobin level to help limit overinvestigation of patients with drug-induced erythrocytosis.
Molecular testing has revolutionized the diagnosis of polycythemia vera, but is associated with substantial costs, ranging from about $350 for JAK2 V617F targeted assays (e.g., qPCR) to $1000 for screening assays by NGS panel. Our findings suggest that increased access to molecular diagnostics has been accompanied by less discriminate use. The high proportion of patients tested for JAK2 mutations with suspected secondary causes of erythrocytosis underscores the importance of a detailed medical history and a medication review to support more judicious use of molecular testing. Medications associated with erythrocytosis, such as testosterone or SGLT2 inhibitors, are common, were used in around 20% of patients in our cohort and are easily identified from the history. In such cases, a diagnostic or therapeutic trial of holding these medications should be considered, with molecular testing reserved for patients who do not respond. Data from our study suggest that stopping SGLT2 inhibitors was associated with a reduction and often normalization of hemoglobin levels, consistent with previous reports.14 Decisions to hold or stop these drugs should be made in collaboration with a patient’s primary care physician or endocrinologist.
Limitations
We conducted this retrospective study at a single tertiary care centre in southwestern Ontario, Canada; practice patterns and access to molecular testing vary across Canada and our findings may not be generalizable. Data included partial years in 2015 and 2021. Our study included patients who had JAK2 mutation testing by either NGS panel or targeted assays; the role of each method in the diagnostic work-up of erythrocytosis remains to be defined, but given the higher cost of NGS, prioritizing targeted assays may offer another means of reducing costs in the investigation of patients with suspected polycythemia vera. We only included patients with elevated hemoglobin levels who underwent JAK2 testing. We did not include all patients referred for erythrocytosis and, thus, were unable to measure a change in volume of referrals or the proportion of referred patients who went on to have molecular testing. Measuring changes in referral volumes — as well as rates of JAK2 testing, stratified by erythrocytosis severity — may have provided a better means of ascertaining the impact of the change in WHO diagnostic criteria on the investigation of suspected polycythemia vera. Although we inferred less discriminate use of molecular testing from the high proportion of patients tested with known secondary causes of erythrocytosis, the presence of secondary causes does not exclude a diagnosis of polycythemia vera, as observed in several patients in our cohort. These findings reinforce the need for an individualized approach to the investigation of erythrocytosis, guided by a focused history (including medication review) and adapted based on the results of ancillary testing and medication adjustments.
Conclusion
Our study showed an increase in JAK2 mutation testing among patients with elevated hemoglobin levels from 2015 to 2021. This increase in molecular testing included a large proportion of patients with known secondary causes of erythrocytosis, many of whom were on SGLT2 inhibitors. These findings can help increase awareness among providers of established and emerging secondary causes of erythrocytosis that are commonly encountered in practice and may be used in the development of more rational approaches to molecular testing, thus improving resource stewardship and lowering health care costs.
Supplementary Material
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
Contributors: Benjamin Chin-Yee and Cyrus Hsia conceived and designed the study. Pratibha Bhai, Michael Levy, Alan Stuart, Hanxin Lin and Bekim Sadikovic analyzed molecular data; Benjamin Chin-Yee, Maxim Matyashin, Ian Cheong, Alejandro Lazo-Langner, Ala Almanaseer, Eri Kawata and Cyrus Hsia collected and interpreted clinical and additional laboratory data. Benjamin Chin-Yee drafted the manuscript. All of the authors revised it critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Data sharing: Data are available on request to the corresponding author, owing to privacy and ethical restrictions.
Supplemental information: For reviewer comments and the original submission of this manuscript, please see www.cmajopen.ca/content/10/4/E988/suppl/DC1.
References
- 1.Kralovics R, Passamonti F, Buser AS, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352:1779–90. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- 2.Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–405. doi: 10.1182/blood-2016-03-643544. [DOI] [PubMed] [Google Scholar]
- 3.Chin-Yee B, Matyashin M, Bhai P, et al. Investigating erythrocytosis: changing practice patterns in the era of molecular diagnostics. Blood. 2021;138(Suppl 1):4630. [Google Scholar]
- 4.Mithoowani S, Laureano M, Crowther MA, et al. Investigation and management of erythrocytosis. CMAJ. 2020;192:E913–8. doi: 10.1503/cmaj.191587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cervi A, Balitsky AK. Testosterone use causing erythrocytosis. CMAJ. 2017;189:E1286–8. doi: 10.1503/cmaj.170683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chin-Yee B, Solh Z, Hsia C. Erythrocytosis induced by sodium-glucose cotransporter-2 inhibitors. CMAJ. 2020;192:E1271. doi: 10.1503/cmaj.76686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gangat N, Szuber N, Alkhateeb H, et al. JAK2 wild-type erythrocytosis associated with sodium-glucose cotransporter 2 inhibitor therapy. Blood. 2021;138:2886–9. doi: 10.1182/blood.2021013996. [DOI] [PubMed] [Google Scholar]
- 8.Zinman B, Wanner C, Lachin JM, et al. EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–28. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 9.Inzucchi SE, Zinman B, Fitchett D, et al. How does empagliflozin reduce cardiovascular mortality? Insights from a mediation analysis of the EMPAREG OUTCOME trial. Diabetes Care. 2018;41:356–63. doi: 10.2337/dc17-1096. [DOI] [PubMed] [Google Scholar]
- 10.Ghanim H, Abuaysheh S, Hejna J, et al. Dapagliflozin suppresses hepcidin and increases erythropoiesis. J Clin Endocrinol Metab. 2020;105:dgaa057. doi: 10.1210/clinem/dgaa057. [DOI] [PubMed] [Google Scholar]
- 11.Mazer CD, Hare GMT, Connelly PW, et al. Effect of empagliflozin on erythropoietin levels, iron stores, and red blood cell morphology in patients with type 2 diabetes mellitus and coronary artery disease. Circulation. 2020;141:704–7. doi: 10.1161/CIRCULATIONAHA.119.044235. [DOI] [PubMed] [Google Scholar]
- 12.Das L, Bhansali A, Walia R. Unmasking and aggravation of polycythemia vera by canagliflozin. Diabet Med. 2018;35:1613–6. doi: 10.1111/dme.13706. [DOI] [PubMed] [Google Scholar]
- 13.Motta G, Zavattaro M, Romeo F, et al. Risk of erythrocytosis during concomitant testosterone and SGLT2-inhibitor treatment: a warning from two clinical cases. J Clin Endocrinol Metab. 2019;104:819–22. doi: 10.1210/jc.2018-01702. [DOI] [PubMed] [Google Scholar]
- 14.Gupta R, Gupta A, Shrikhande M, et al. Marked erythrocytosis during treatment with sodium glucose cotransporter-2 inhibitors-report of two cases. Diabetes Res Clin Pract. 2020;162:108127. doi: 10.1016/j.diabres.2020.108127. [DOI] [PubMed] [Google Scholar]
- 15.Brown E, Heerspink HJL, Cuthbertson DJ, et al. SGLT2 inhibitors and GLP-1 receptor agonists: established and emerging indications. Lancet. 2021;398:262–76. doi: 10.1016/S0140-6736(21)00536-5. [DOI] [PubMed] [Google Scholar]
- 16.O’Meara E, McDonald M, Chan M, et al. CCS/CHFS heart failure guidelines: clinical trial update on functional mitral regurgitation, SGLT2 inhibitors, ARNI in HFpEF, and tafamidis in amyloidosis. Can J Cardiol. 2020;36:159–69. doi: 10.1016/j.cjca.2019.11.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.