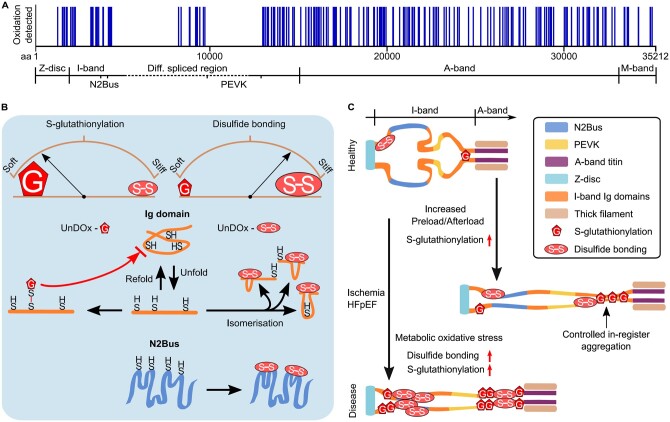
Figure 4.
Titin oxidation sites and mechanisms of how S-glutathionylation and disulphide bonding alter titin stiffness in health and disease. (A) Titin oxidation sites detected in mouse heart tissue by mass spectrometry (data obtained from Ref.76). (B) Changes in titin stiffness are dependent on whether more S-glutathionylation or more disulphide bonding occurs in unfolded I-band titin domains. Increased compliance is mediated via the S-glutathionylation mechanism involving Ig domains and increased stiffness via the disulphide bonding mechanism involving Ig domains or the N2Bus. Brown arches depict titin spring stiffness. (C) Observed changes in I-band titin oxidation under cardiac stress (increased preload/afterload) or in diseased states (ischaemia, HFpEF) modulate titin stiffness in a complex manner. S-glutathionylation under cardiac stress can cause controlled in-register aggregation of distal I-band titin, whereas disease states can be associated with a general increase in titin oxidation. aa, amino acid; HFpEF, heart failure with preserved ejection fraction; Ig, immunoglobulin-like; UnDOx, unfolded domain oxidation.