Abstract
In the past few years, numerous advances emerged in terms of circulating microRNA(miRNA) regulating gene expression by circulating blood to the distal tissues and cells. This article reviewed and summarized the process of circulating miRNAs entering the circulating system to exert gene regulation, especially exogenous miRNAs (such as plant miRNAs), from the perspective of the circulating miRNAs source (cell secretion or gastrointestinal absorption), the transport form and pharmacokinetics in circulating blood, and the evidence of distal regulation to gene expression, thereby providing a basis for their in-depth research and even application prospects.
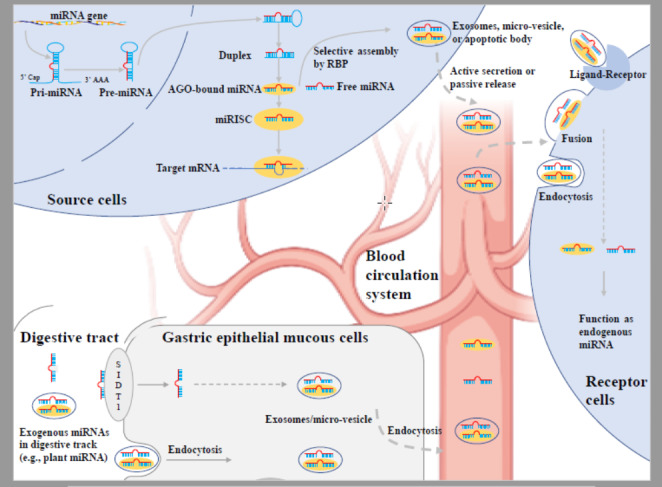
Graphical Abstract
Keywords: Circulating microRNA, Plant microRNA, Distal regulation, Gastrointestinal absorption, Post-translational regulation
Introduction
MicroRNAs (miRNAs) are a class of small, single-stranded endogenous non‐coding RNA comprising ~ 22 nucleotides (nt) that processed from a stem-loop structured precursor transcript (Kim 2005). MiRNAs exert their affects via base complementary pairing with target message RNA (mRNA), degrading corresponding mRNA or suppressing mRNA translation, as well as act as fine-tuners of the expression of mRNAs (Bartel and Chen 2004). In 2008, Chen et al. found that there were a large number of miRNAs stably existing in human plasma and serum that could circulate to recipient cells and may play a role in regulating gene expression, termed as circulating miRNAs (Chen et al. 2008). Moreover, the circulating miRNA has been demonstrated to own unusually high stability is able to remain stable under various extreme conditions, such as boiling, a very low or high pH, repeated freeze and thaw, and storage at room temperature for a long time (Chen et al. 2008). This feature assures the miRNAs enter and stably exist in the circulatory system of the body and then reach the receptor cells to play a distal regulatory role. In addition, miRNAs were also found in other body fluids such as saliva (Park et al. 2009), urine (Hanke et al. 2010), breast milk (Kosaka et al. 2010), but these miRNAs are outside the scope of this paper.
Since researchers first discovered some dietary-derived plant miRNAs stably in human blood in 2011 (Zhang et al. 2012), the academic community has carried out a series of studies and made significant progress on the biological process that how a circulating miRNA originates from cells or plant food enters the circulatory system, circulate to receptor cells, and play a distal regulatory role in the gene expression. The present article performed a review study by dividing the process into the circulating miRNAs source (cell secretion or gastrointestinal absorption), the transport form and pharmacokinetics in circulating blood, and the evidence to function as distal regulators for gene expression, aiming to demonstrate the feasibility of exogenous miRNA as a new active substance entering the receptor cells of the body to play a regulatory role, and to provide scientific basis for subsequent development, research and application prospects.
Intracellular miRNA synthesis and gene regulation
Typically, miRNA is synthesized by transcription of cell genes. The process of miRNA biogenesis in animal cells involves the following steps. First of all, genes are transcribed as primary transcripts (pri-miRNAs) containing stem-loop structures in the nucleus. Next, the stem-loop structure in pri-miRNA will be cut by the endonuclease Drosha, resulting in a length of about 70nt precursor miRNA (pre-miRNAs). The pre-miRNA is then transported from the nucleus by Exportin-5 into the cytoplasm and the “loop” structure is further cleaved by Dicer/TRBP (TAR RNA-binding protein) to generate mature miRNA/miRNA* duplex. The mature miRNA is then loaded onto Argonautes (Ago) to form the core effector complexes, known as miRNA-induced silencing complexes (miRISCs), whereas the miRNA* strand in the duplex undergoes unwinding, shedding, and degradation (Iwakawa and Tomari 2022). In plants, the biological process of miRNA is different from that in animals. In plants, the cleavage of pri-miRNA is performed by Dicer-like protein (DCL) due to the lack of Drosha homologous proteins, generating pre-miRNA with hundreds of nt in length (Liang et al. 2014). Coincidentally, the cleavage of pre-miRNA is also performed by Dicer-like protein, and both the cleavage of pri-miRNA and pre-miRNA are occurred in the nucleus. After transport to the cytoplasm, the 3’ terminal riboses of plant miRNA undergoes methylation by Hua enhancer 1 (HEN1), resulting in the stability of plant miRNAs against strong acid, strong alkali, and high temperature, which lays a molecular biological foundation for its entry into the organism through the digestive tract (Yu et al. 2005).
In animal or human cells, miRNAs bind target mRNA sequences mainly through the canonical base pairing between the seed sequence, which includes nucleotides 2–8 from the 5′-end, and the complementary sequence found in the 3′ untranslated region (3′UTR) of its target mRNA, leading to transcriptional inhibition (Fabian et al. 2011), cleavage (Yekta et al. 2004) or degradation (Wu et al. 2006) of target mRNA. In-depth study of miRNA has illustrated that it also targeted bind to the 5’UTR (Jopling et al. 2008; Orom et al. 2008) and open reading frame (ORF) (Bartel 2004), to play the role of gene regulation. In common with animal miRNAs, plant miRNAs exert their affects via base complementary pairing with target gene mRNA after entering animal or human cells (Cavalieri et al. 2016; Chin et al. 2016; Hou et al. 2018). MiRNAs have been estimated to be involved in about 1/3 of human gene expression regulation, covering many aspects of cellular behavior such as cell growth, division, differentiation, proliferation, apoptosis, and metabolism, and are an important class of gene regulatory molecules (Lewis et al. 2005; Chen et al. 2006).
Source of circulating miRNA
In vivo cell release and related mechanisms
Part of the miRNA synthesized in cells can be actively secreted into the blood circulation, which increases the content of the corresponding circulating miRNAs and reaches the receptor cells to perform distal regulatory functions. This phenomenon is most prominent during special physiological and pathological periods, like pregnancy, intestinal flora changes, pathogenic microorganism infection, and tumor occurrence. There is evidence that the expressions of miR-516-5p, miR-518b, miR-520 h, miR-525, and miR-526a in the plasma of pregnant women are up-regulated and increase with the progress of pregnancy, and eventually return to basal levels after delivery (Gilad et al. 2008; Gunel et al. 2011; Kotlabova et al. 2011). The above-mentioned circulating miRNAs can pass the placental barrier and affect fetal development (Li et al., 2015). Host miRNAs or food miRNAs may enter the gut bacteria and affect the growth and reproduction of gut flora (Liu et al. 2016, 2019; Teng et al. 2018). On the contrary, the disturbance of intestinal flora may lead to the change of miRNA expression profile in the host circulation (Peck et al. 2017; Moloney et al. 2018; Virtue et al. 2019; Zhu et al. 2020a, b), exerting a regulatory role in the physiological function of the body. Generally, virus infection often induces the differential expression of host cells and secretes signature miRNA (Xu et al. 2021), which is an important source of circulating miRNA in the infected state and contributes to the repertoire of virus-host interactions (Gonda et al. 2019; Zhu et al. 2020a, b). For example, Epstein Barr virus (EBV) can specifically express viral miRNA in B cells, such as miR-BART15, which is secreted by cells through exosomes and transferred to uninfected cells, inhibiting the expression of its binding nucleotide binding oligomerization domain like receptor protein 3 (NLRP3), thereby increasing the susceptibility of B cells to the virus (Pegtel et al. 2010). During tumorigenesis, tumor cells, stromal cells and endothelial cells exposed to the tumor microenvironment can secrete miRNAs through micro-vesicles (Yin et al. 2014) and exosomes (Zhang et al. 2015; Liang et al. 2016; Sun et al. 2018), thereby significantly changing the expression profile of circulating miRNAs. Tumor cells secrete exosomes at least 10-fold more than normal cells (Sun et al. 2018), and the miRNA carried by tumor cell-derived exosomes can enter the circulatory system to play a distal regulatory role as a carcinogen or tumor suppressor (Selth et al., 2012; Chin et al. 2016). For example, miR-222 is highly expressed in exosomes derived from tumors (Di Leva and Croce, 2010; Mao et al. 2018), the expression of miR-222 in the plasma of breast cancer patients is significantly higher than that of normal people, furthermore, the expression of miR-222 in breast cancer patients with lymphatic metastasis is higher than that in non-metastatic group (Ding et al. 2018). Other pathological tissue cells may also lead to significant changes of circulating miRNA (Zhang et al. 2010; Kimura et al. 2018). The expression levels of these characteristic circulating miRNAs have clinical implications for disease diagnosis, prognosis, and even treatment.
MiRNAs are mainly secreted into the circulatory system by cells in the form of encapsulated exosomes or micro-vesicles. The secretion of miRNA by cells in the body has been proved to be selective (Squadrito et al. 2014; Garcia-Martin et al. 2022). RNA binding protein (RBP) holds an irreplaceable position in the selective secretion of miRNA, which can regulate the loading of specific miRNA into extracellular exosomes or micro-vesicles through various mechanisms, and finally achieve the purpose of regulating the secretion of miRNA (Groot and Lee 2020), as shown in Table 1. Currently, the underlying mechanism of many RBPs regulating miRNA remains elusive. In addition, studies have shown that the selective secretion of miRNAs by cells is affected by the expression ratio of intracellular miRNAs to target mRNAs (miRNA/mRNA). Specifically, a small miRNA/mRNA ratio favors miRNA residency in cells, and vice versa favors miRNA exocytosis, thereby ensuring moderate regulation of target mRNAs (Squadrito et al. 2014).
Table 1.
The regulatory effect of RNA binding protein on miRNA secretion
| Name of RBP | Regulated miRNA | Effect on miRNA’s secretion | Regulatory mechanism |
|---|---|---|---|
| hnRNP-A2B1 | miR-198̖;miR-601 | Promoting | Specifically binding to GGAG in miRNA, leading it to exosomes (Villarroya-Beltri et al. 2013) |
| Promoting | RRM1 Specifically binding to AGG in miRNA, leading it to micro-vesicles, specifically binding to UAG in miRNA, leading it to micro-vesicles (Wu et al. 2018) | ||
| miR-503 | Inhibiting | hnRNPA2B1 migrates into the nucleus and cannot bind to miRNAs (Perez-Boza et al. 2020) | |
|
hnRNP-Q (SYNCRIP) |
miR-3470a̖;miR-194-2-3p | Promoting | Specifically binding to GGCU in miRNA, leading it to exosomes (Santangelo et al. 2016) |
| AGO2 | let-7a̖;miR-100̖;miR-320a | Inhibiting | Phosphorylation of Ago2 on serine 387 reduces Ago2 secretion into exosomes, preventing exosomal secretion of AGO2-bound miRNAs (McKenzie et al. 2016) |
| Y-box protein 1 | miR-223 | Promoting | Promoting the miRNA loaded into exosomes (Shurtleff et al. 2016) |
| miR-133 | Promoting | Promoting the miRNA loaded into exosomes (Lin et al. 2019) | |
| MEX3C | miR-451a | Promoting | MEX3C/AP-2 Recruiting the miRNA to endosomes for subsequent exosomal sorting with the aid of AGO2(Lu et al. 2017) |
| MVP | miR-193a | Promoting | Not interpreted (Teng et al. 2017) |
| La protein | miR-122 | Promoting | Not interpreted (Temoche-Diaz et al. 2019) |
| nSMase2 | miR-210 | Promoting | Not interpreted (Kosaka et al. 2013) |
| hnRNP-K̖;SAFB | EV-associated miRNAs during autophagy | Promoting | Through LC3-conjugation pathway promot the miRNA loaded into lipid LC3-rich EVs(Leidal et al. 2020) |
| miR-486 | 3’-adenylylated miRNAs tend to reside within cells, and 3’-urididated miRNAs tend to be released exosomes (Koppers-Lalic et al. 2014) |
hnRNP: Heterogeneous Nuclear Ribonucleoproteins; hnRNP-Q(SYNCRIP): Synaptotagmin-binding cytoplasmic RNA-interaction protein; AO2: Argonaute 2; MEX3C: RNA-binding ubiquitin E3 ligase;MVP:Major vault protein;nSMase2:Neural Sphingomyelinase 2;SAFB:scaffold-attachment factor B
In addition to active secretion, apoptotic bodies formed during normal cell metabolism can also carry miRNAs into the circulatory system. For example, vascular endothelial cells can release miR-126 into the blood through apoptotic bodies once the body develops atherosclerosis (Zernecke et al. 2009). In the case of tissue cell damage and necrosis, its miRNA can also be passively released into the circulatory system. For example, a large number of cardiomyocytes miR-208 and miR-499 are passively released into the circulatory system during acute myocardial infarction, increasing their plasma concentrations by 1600- and 100-fold, respectively (Corsten et al., 2012).
Digestive tract absorption and related mechanisms
Another important source of circulating miRNAs is the absorption of exogenous miRNAs (such as plant miRNAs) into the blood through the digestive tract. Zhang et al. (2012) first found that about 5% of miRNAs in human and animal serum have anti-sodium periodate properties in 2011, and thus speculated that their possible sources were plant miRNAs in daily diet, and further proposed the view that plant miRNAs in food could be absorbed into the body’s blood circulation through digestive tract. The research team subsequently confirmed through experiments that mice miR-168a was absorbed into the blood circulation through the digestive tract and reached the liver, simultaneously targeted the liver low-density lipoprotein receptor adaptor protein 1 (LDLRAP1) and inhibited its expression, ultimately promoted the metabolism of low-density lipoprotein (LDL) (Zhang et al. 2012). Since then, the view that exogenous plant miRNAs can enter the blood through the digestive tract and reach distant tissues and organs in the body has been continuously confirmed by other studies. Liang et al. detected cabbage miRNA in the gastrointestinal, blood, spleen, liver, kidney, feces, and other samples of mice after feeding mice with cabbage (Liang et al. 2014). Liang et al. recruited volunteers to eat watermelon juice or mixed fruits and detected a variety of corresponding fruit miRNAs in their plasma (Liang et al. 2015). Luo et al. detected 16 maize miRNAs in the serum, pancreas, and longissimus dorsi of pigs after feeding fresh maize for 7 days (Luo et al. 2017). In addition, Tarallo et al. found that there were significant differences in the expression profiles of fecal miRNA among vegans, vegetarians, and omnivores, indirectly indicating the influence of food miRNA on human body (Tarallo et al. 2021). Based on the above argument and evidence, the researchers further studied the absorption of herbal miRNA in the body, successfully found that herbal miRNA could still exist stably after being decocted at high temperature and was able to be absorbed into the blood through the digestive tract, as well as played a regulatory role in the distal tissues of the body (Li et al., 2015; Zhou et al. 2015; Zhou et al. 2020; Kalarikkal and Sundaram, 2021; Teng et al. 2021). Besides plant miRNAs, miRNAs in milk exosomes could also be absorbed into the blood through the digestive tract (Manca et al. 2018). Moreover, our body might obtain circulating miRNA by in vitro injection as well, making sense for its applicable usage (Teng et al. 2021).
Some plant or herbal miRNAs have been confirmed by existing studies to have strong heat, strong acid, and other stability, and can withstand high-temperature cooking, torment, and digestion and degradation. The reasons can be attributed to the following aspects: (1) Methylation at the 3’ end of plant miRNAs (Zhang et al. 2012) and/or its special sequence composition (such as rich in CG) (Zhou et al. 2015); (2) Plant cell exosomes have strong thermal and acid stability (Lasser et al. 2011; Mu et al. 2014), which can effectively protect their encapsulated miRNA. The differences in the expression profiles of plant miRNAs in food and plasma indicate that the body also selectively absorbs plant miRNAs (Zhang et al. 2010). Based on the synthesis and action process of miRNA, it is speculated that the existing forms of miRNA in food include pri-miRNA, pre-miRNA, miRNA:miRNA*duplex, free miRNA, AGO2-bound miRNA, miRISC. Studies have shown that mature free miRNA (Zhang et al. 2012) and miRNA:miRNA*duplex (Chin et al. 2016; Hou et al. 2018) can be absorbed into the blood and become circulating miRNAs. Two mechanisms may be involved in the entry of exogenous plant miRNAs into the body’s circulation. First, plant miRNAs are absorbed by gastrointestinal epithelial cells, and then actively secreted into the blood by gastrointestinal epithelial cells (Jia et al. 2021). Chen et al. demonstrated through in vitro and in vivo experiments that dietary plant miRNAs can be absorbed into the blood by SID-1 transmembrane family member 1 (SIDT1) in the plasma membrane of gastric epithelial mucous cells (Chen et al. 2021). Second, plant miRNAs are encapsulated in plant exosomes and absorbed by gastrointestinal epithelial cells through endocytosis (Kusuma et al. 2016; Manca et al. 2018); and then actively secreted into the blood by gastrointestinal epithelial cells.
Transport forms and pharmacokinetics of miRNAs in circulating blood
Circulating miRNAs are characterized by high stability, and one of the important reasons for this characteristic is that they are usually encapsulated and transported by exosomes, micro-vesicles, apoptotic bodies, and other vesicles (A et al., 2009; Blanc and Vidal 2010), thus reducing or avoiding the degradation of various nucleic acid metabolizing enzymes. There is evidence that up to 83–99% of circulating miRNAs are stored in exosomes (Gallo et al. 2012); miRNAs in exosomes include AGO2-bound miRNAs, free miRNA, mature miRISCs (Mao et al. 2015). Another important reason is that circulating miRNAs inside and outside vesicles are often bound to proteins, which can also improve their stability. Studies have shown that 90% of circulating miRNAs are bound to RBPs (Chang et al. 2004; Arroyo et al. 2011), such as AGO2 protein (Arroyo et al. 2011), high density lipoprotein (HDL) (Vickers et al. 2011; Tabet et al. 2014) and LDL (Wagner et al. 2013), and most circulating miRNAs are transported as AGO2-bound miRNAs (Arroyo et al. 2011).
The metabolism of circulating miRNAs in blood is likewise a significant parameter. In theoretical terms, plant miRNA in food is absorbed through the gastrointestinal tract, and its content level first increases in the gastrointestinal tissue, and then enters the blood circulation to become circulating miRNA, which is selectively absorbed by distant tissue cells. Zhang et al. (2012) showed that the content level of MIR-168a in plasma and liver tissue increased significantly at 6-hour after mice ate fresh rice. Further research found that the content level of MIR-168a in plasma and liver tissue reached the peak 3-hour after mice were intervened by total RNA extract of rice (Zhang et al. 2012). Hou et al. found that serum MIR-156a peaked within 1 to 3 h in most of the five healthy volunteers eating lettuce (Hou et al. 2018). Additional studies have shown that the plasma MIR-2911 of animals reached the peak after continuous feeding of honeysuckle for 3 days and returned to the baseline level 2-day after stopping feeding (Yang et al. 2015a, b). After oral administration of honeysuckle decoction, the content of MIR-2911 in plasma and lung tissue of mice increased and reached the peak at 6 h, followed by a gradual decline, eventually the lung MIR-2911 recovered to the baseline level at 12 h (Zhou et al. 2015). Furthermore, it is reported that MIR-2911 could be detected in the serum of male ICR mice 5-minute after tail vein injection of an equal mixture of MIR-2911, MIR-168a, MIR-156a and MIR-161, and all miRNAs were cleared after 3 h (Yang et al. 2015a, b). All the data above indicates that circulating miRNAs can exist in the body for hours or days (Ruegger and Grosshans 2012), and their levels can be kept stable through continuous feeding. It is worth noting that intravenous injection can rapidly increase circulating miRNA content (Yang et al. 2015a, b).
Evidence to function as distal regulators for gene expression
Recent research demonstrates that circulating miRNAs, especially exogenous plant miRNAs that are absorbed into the blood, can enter receptor cells through endocytosis, membrane fusion or ligand-receptor binding (Cavalieri et al. 2016; Chin et al. 2016; Hou et al. 2018), and play a distal role to regulate gene expression. Table 2 lists the research reports about researchers intervening in cells or organisms by molecular biology techniques in recent years so that exogenous miRNAs can enter recipient cells and play a regulatory role in cells or organisms. These identified studies amply illustrate that circulating miRNAs, especially exogenous plant miRNAs, can overcome multiple barriers to enter the body, reach recipient cells, and perform transboundary gene regulation functions.
Table 2.
Evidence of miRNAs to function as distal regulators for gene expression
| miRNA Source | miRNA ID |
Studied objects | Administrating/ Intervening methods | Corresponding change of expression level in target issue/cells | Observed functions | References |
|---|---|---|---|---|---|---|
| Endogenous miRNA | ||||||
| MV secreted by u87-MG astrocytes | miR-34a | SH-SY5Y cell/ 6-OHDA model rats | Co-culture with MV/inject | miR-34a was significantly increased in SH-SY5Y cells/unclear | Targeting anti-apoptotic Bcl-2 proteins,enhances the sensitivity of dopaminergic neurons to neurotoxins and accelerates neuronal injury | (Mao et al. 2015) |
| Human cytomegalovirus |
miR- UL148D |
Kasumi-3 cell and CD34 + hematopoietic stem cells |
Co-culture with strain NR-1 | Significantly increase | Target IER5 and promote viral latency through iER5-CDC25B-CDK1 signaling pathway | (Pan et al. 2016) |
|
MV secreted by Lewis lung cancer |
miR-214 | CD4+T cell /BALB/c nude mice | Co-culture/tail vein injection with fluorescent labeled MV | Significantly increase/Fluorescence markers were found in nude mice | Target PTEN.down-regulate PTEN-mediated signal cascade to promote Treg cell amplification and tumor growth | (Yin et al. 2014) |
| Exsome secreted by MCF-7 and MDA-MB-231 cell | miR-222 | MCF-7 cell/ Female nude mice | Co-culture with exosomes/subcutaneous injection | Confocal microscopy images indicate exosome uptake / unclear | Target NF-KB promotes cell invasion and migration, and promotes tumor growth | (Ding et al. 2018) |
| Exsome secreted by HCT-116 and SW480 | miR-200b |
HCT-116 and SW480 cell/BALB/c males mice |
Co-culture with Exo-miR-200b/inject | Significantly increase/Immuno fluorescence analysis increased | Target P27 and RND3, promote colorectal cancer growth | (Zhang et al. 2018) |
| Exsome secreted by HCC cell | miR-21 | LX2 cell/Female nude mice | Co-culture with exosomes/Caudal vein injection | increase/unclear | activate HSC through the PTEN/PDK1/AKT signaling axis,promote liver fibrosis, tumor growth and angiogenesis | (Zhou et al., 2018) |
| Exsome secreted by MCF-7̖;HCC-1937 and MDA-MB-231 cells | miR‑7641 | MCF-7̖;HCC-1937̖;MDA-MB-231̖;Primary breast cancer cell/BALB/c Female nude mice | Transfection/intravenous injection | increase/unclear | Promote breast tumor cell proliferation, increase its invasiveness, promote tumor metastasis | (Shen et al. 2021) |
| Herbs | ||||||
| HS decoction̖;Synthetic miR-2911 | miR-2911 | MDCK cell /BALB/c mice | Co-culture with decoction total RNA or synthetic miR-2911/gavage | unclear/ Plasma, lung and liver tissues of mice were detected | Inhibit the replication of H1N1, H5N1 and H7N9 viruses and significantly reduce the mortality caused by virus infection | (Zhou et al. 2015) |
| HS decoction | miR-2911 | Pregnant mice | gavage | Plasma, placenta and fetal liver of pregnant mice miR2911 Significantly increase | Regulate fetal growth and development | (Li et al. 2015) |
| Exosomes from cells transfected with miR-2911 or HS decoction | miR-2911 | Vero E6 cell/ Healthy volunteers | Co-culture with exosome/ oral | miR-2911 was elevated in Vero E6 cells and serum of volunteers | Inhibit novel Coronavirus SARS-COV-2 replication and accelerate negative conversion in patients with COVID-19 | (Zhou et al. 2020) |
| Synthetic astragalus miR-396 | miR-396 | BALB/ c asthmatic model mice | Nasal feeding | Peripheral blood, spleen and lung were significantly increase | Inhibit the expression of GATA-3 in Th2 cells | (Shen et al. 2019) |
| Plant food | ||||||
| Synthetic single strand mature miR-168a and fresh rice | miR-168a | HepG2 cell/mice | Co-culture with synthetic miR-168a/oral feeding | miR-168a was significantly elevated in HepG2 cells and mice | Target LDLRAP1 and promote LDL clearance | (Zhang et al. 2012) |
| Strawberry | FvmiR-168 | Dendritic cell | Co-culture with FvmiR-168 | increase | Strawberry miRNA acts on the Toll-like receptor of dendritic cells and inhibits their migration, thereby reducing inflammation | (Cavalieri et al. 2016) |
| MV secreted by healthy persons; Synthetic miR-159 | miR-159 | MDA-MB-231 cell /NSG mice | Co-culture with MV/ gavage | unclear/miR-159 was detected in the gastrointestinal tract | Target TCF7,inhibits breast tumor cell proliferation | (Chin et al. 2016) |
| Synthetic miR-156a | miR-156a | CNE2̖;HONE1̖;C666-1 cell | Co-culture with miR-156a mimic | unclear | Target JAMA,inhibit the development of nasopharyngeal carcinoma | (Tian et al. 2016) |
| vegetables | miR-156a | HAEC cell | Co-culture with miR-156a | Significantly increase | Inhibit JAMA expression in human aortic endothelial cells and reduce monocyte adhesion | (Hou et al. 2018) |
| Rape pollen | miR-162a | Bee larvae | oral feeding | miR-162a was detected in vivo | Target mTOR,delay the development of young bees, reducing body and ovarian size, and promoting their development into worker bees | (Zhu et al. 2017) |
| ELNs original ginger | aly-miR-396a-5p | Vero E6 cell / septic mouse model | Co-culture with aly-miR-396a-5p/Intratracheal injection | increase/ Fluorescence appeared in lung and serum of mice |
Inhibit the expression of Novel Coronavirus spike protein and NSP12 protein respectively, reduce inflammation and apoptosis |
(Teng et al. 2021) |
| Synthetic miR-159(rich in soybean) | gma-miR-159a | Hepatic stellate cell/hepatic fibrosis model ICR mice | Co-culture with fluorescent-labeled miR-159a/intraperitoneal injection | Fluorescent markers were observed either intracellular or in vivo | Target GSK-3β, inhibit the expression of GSK-3β and its downstream protein, alleviati liver fibrosis | (Yu et al. 2021) |
HS: Honeysuckle; NSG: NOD-scid IL2Rgnull; HAEC cell: human aortic endothelial cell; JAMA: junction adhesion molecule-A; ELNs: exosome-like nanoparticles
Discussion and future perspective
Taken together, endogenous miRNA and exogenous plant miRNA can enter the body’s blood circulation through cell excretion or gastrointestinal absorption, correspondingly causing changes of circulating miRNA expression profile, as well as enter the distal tissue receptor cells to play a role in regulating gene expression. The above-mentioned findings provide significance for the application prospects of miRNAs, especially plant miRNAs. Currently, circulating miRNA has been used as a biomarker for early screening, diagnosis, and prognosis of diseases (Takahashi et al. 2019) because of its stable existence in body fluids such as plasma and serum, state-specific differences in expression profiles in different physiological and pathological states, convenient sampling, and sensitive detection. Moreover, the long-range regulatory properties of miRNA make it potential for gene-targeted therapy. For the moment, miRNA drugs for targeted therapy of leukemia have entered phase I and phase II clinical trials (Takahashi et al. 2019). How to deliver exogenous miRNA drugs to target tissue cells for targeted therapy is a key technology to improve the effect of miRNA targeted therapy on cancer (Sabit et al. 2021) and also a difficulty in the development and utilization of miRNA drugs. In recent years, viruses are generally considered to be the first-choice for miRNA delivery due to their advantages of strong specificity, high delivery efficiency, low off-target effects, and long expression duration (Havlik et al., 2020). Some plants are rich in miRNAs with specific functions (Cavalieri et al. 2016; Zhu et al. 2017; Hou et al. 2018; Teng et al. 2021), and can introduce miRNAs with therapeutic effects into the body using natural vegetables or herbs as carriers. This type of miRNA delivery method is low-toxic and economical, which is considered to be a promising research and development direction.
Although existing studies have proved that circulating miRNAs, especially exogenous plant miRNAs, can overcome many barriers to reach the receptor cells and exert the function of remote gene regulation (Cavalieri et al. 2016; Zhu et al. 2017; Hou et al. 2018; Teng et al. 2021), the molecular biological processes of plant miRNAs, including selective uptake and secretion, entry into recipient cells, and formation of mature miRISCs, are still poorly understood. As outlined previously, during the assembly of intracellular miRNAs into miRISCs, mature miRNAs are loaded onto AGO proteins in the form of miRNA:miRNA*duplex to form miRISC precursors, and mature miRISCs are formed after miRNA* unwinds and falls off. However, in the current functional studies on the introduction of exogenous miRNAs into the body or cells, the initial miRNA interveners used involve many forms, including mature free miRNA monomer (Zhang et al. 2012), miRNA: miRNA* dimer (Chin et al. 2016), exosomes (Shen et al. 2021) or natural plants (Hou et al. 2018) containing target miRNA, pre-miRNA (Almanza et al. 2018). Here are some key issues to be further interpreted. What is the difference between the above-mentioned exogenous miRNA interveners and intracellular miRNAs during their assemblies of miRISC? Which exogenous miRNA interveners have better distal regulatory effect? And how to artificially synthesize exogenous miRNA interveners with good stability and high absorption rate for subsequent application? All these are important issues to be solved in the process of miRNA drug development. In conclusion, circulating miRNAs, originated from cell excretion or gastrointestinal absorption, can overcome barriers to reach the receptor cells and exert the function of remote gene regulation. While the detail mechanisms are still waiting to be interpreted.
Author contributions
Conceptualization, TLX and XZ, writing and original draft preparation, QNW; review and editing, WQN, LXL, YJ, TLX, and XZ; design of tables, TLX and XZ; funding acquisition, TLX. All authors have read and agreed to the published version of the manuscript.
Funding
This review is supported by National Natural Science Foundation of China grant (NO. 82141214), Jiangxi Provincial Department of Education (NO. GJJ211212), and Jiangxi Provincial Administration of Traditional Chinese Medicine (NO. 2020A0312) to TLX, and Jiangxi University of Chinese Medicine 1050 Youth Talent Project (NO. 5142001011) to XZ.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Qingni Wu and Longxue Li contributed equally to this work.
Contributor Information
Tielong Xu, Email: xutielong@jxutcm.edu.cn.
Xu Zhou, Email: zhouxu_ebm@hotmail.com.
References
- Almanza G, Rodvold JJ, Tsui B, et al. Extracellular vesicles produced in B cells deliver tumor suppressor miR-335 to breast cancer cells disrupting oncogenic programming in vitro and in vivo. Sci Rep. 2018;8:17581. doi: 10.1038/s41598-018-35968-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arroyo JD, Chevillet JR, Kroh EM, et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci U S A. 2011;108:5003–5008. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Bartel DP, Chen CZ. Micromanagers of gene expression: the potentially widespread influence of metazoan microRNAs. Nat Rev Genet. 2004;5:396–400. doi: 10.1038/nrg1328. [DOI] [PubMed] [Google Scholar]
- Blanc L, Vidal M. Reticulocyte membrane remodeling: contribution of the exosome pathway. Curr Opin Hematol. 2010;17:177–183. doi: 10.1097/MOH.0b013e328337b4e3. [DOI] [PubMed] [Google Scholar]
- Cavalieri D, Rizzetto L, Tocci N, et al. Plant microRNAs as novel immunomodulatory agents. Sci Rep. 2016;6:25761. doi: 10.1038/srep25761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J, Nicolas E, Marks D, et al. miR-122, a mammalian liver-specific microRNA, is processed from hcr mRNA and may downregulate the high affinity cationic amino acid transporter CAT-1. RNA Biol. 2004;1:106–113. doi: 10.4161/rna.1.2.1066. [DOI] [PubMed] [Google Scholar]
- Chen JF, Mandel EM, Thomson JM, et al. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat Genet. 2006;38:228–233. doi: 10.1038/ng1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Zhang F, Dong L, et al. SIDT1-dependent absorption in the stomach mediates host uptake of dietary and orally administered microRNAs. Cell Res. 2021;31:247–258. doi: 10.1038/s41422-020-0389-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Ba Y, Ma L, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18:997–1006. doi: 10.1038/cr.2008.282. [DOI] [PubMed] [Google Scholar]
- Chin AR, Fong MY, Somlo G, et al. Cross-kingdom inhibition of breast cancer growth by plant miR159. Cell Res. 2016;26:217–228. doi: 10.1038/cr.2016.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsten MF, Dennert R, Jochems S, et al. Circulating MicroRNA-208b and MicroRNA-499 reflect myocardial damage in cardiovascular disease. Circ-Cardiovasc Gene. 2012;3:499–506. doi: 10.1161/CIRCGENETICS.110.957415. [DOI] [PubMed] [Google Scholar]
- Di Leva G, Croce CM. Roles of small RNAs in tumor formation. Trends Mol Med. 2010;16:257–267. doi: 10.1016/j.molmed.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J, Xu Z, Zhang Y, et al. Exosome-mediated miR-222 transferring: An insight into NF-κB-mediated breast cancer metastasis. Exp Cell Res. 2018;369:129–138. doi: 10.1016/j.yexcr.2018.05.014. [DOI] [PubMed] [Google Scholar]
- Fabian MR, Cieplak MK, Frank F, et al. miRNA-mediated deadenylation is orchestrated by GW182 through two conserved motifs that interact with CCR4-NOT. Nat Struct Mol Biol. 2011;18:1211–1217. doi: 10.1038/nsmb.2149. [DOI] [PubMed] [Google Scholar]
- Gallo A, Tandon M, Alevizos I, et al. The majority of microRNAs detectable in serum and saliva is concentrated in exosomes. PLoS ONE. 2012;7:e30679. doi: 10.1371/journal.pone.0030679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Martin R, Wang G, Brandao BB, et al. MicroRNA sequence codes for small extracellular vesicle release and cellular retention. Nature. 2022;601:446–451. doi: 10.1038/s41586-021-04234-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilad S, Meiri E, Yogev Y, et al. Serum microRNAs are promising novel biomarkers. PLoS ONE. 2008;3:e3148. doi: 10.1371/journal.pone.0003148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonda A, Kabagwira J, Senthil GN, et al. Internalization of Exosomes through Receptor-Mediated Endocytosis. Mol Cancer Res. 2019;17:337–347. doi: 10.1158/1541-7786.MCR-18-0891. [DOI] [PubMed] [Google Scholar]
- Groot M, Lee H (2020) Sorting Mechanisms for MicroRNAs into Extracellular Vesicles and Their Associated Diseases. Cells 9. 10.3390/cells9041044 [DOI] [PMC free article] [PubMed]
- Gunel T, Zeybek YG, Akcakaya P, et al. Serum microRNA expression in pregnancies with preeclampsia. Genet Mol Res. 2011;10:4034–4040. doi: 10.4238/2011.November.8.5. [DOI] [PubMed] [Google Scholar]
- Hanke M, Hoefig K, Merz H, et al. A robust methodology to study urine microRNA as tumor marker: microRNA-126 and microRNA-182 are related to urinary bladder cancer. Urol Oncol. 2010;28:655–661. doi: 10.1016/j.urolonc.2009.01.027. [DOI] [PubMed] [Google Scholar]
- Havlik LP, Simon KE, Smith JK, et al. Co-evolution of AAV capsid antigenicity and tropism through a structure-guided approach. J Virol. 2020;94:e00976–e00920. doi: 10.1128/JVI.00976-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou D, He F, Ma L, et al. The potential atheroprotective role of plant MIR156a as a repressor of monocyte recruitment on inflamed human endothelial cells. J Nutr Biochem. 2018;57:197–205. doi: 10.1016/j.jnutbio.2018.03.026. [DOI] [PubMed] [Google Scholar]
- Iwakawa HO, Tomari Y. Life of RISC: Formation, action, and degradation of RNA-induced silencing complex. Mol Cell. 2022;82:30–43. doi: 10.1016/j.molcel.2021.11.026. [DOI] [PubMed] [Google Scholar]
- Jia M, He J, Bai W, et al. Cross-kingdom regulation by dietary plant miRNAs: an evidence-based review with recent updates. Food Funct. 2021;12:9549–9562. doi: 10.1039/d1fo01156a. [DOI] [PubMed] [Google Scholar]
- Jopling CL, Schutz S, Sarnow P. Position-dependent function for a tandem microRNA miR-122-binding site located in the hepatitis C virus RNA genome. Cell Host Microbe. 2008;4:77–85. doi: 10.1016/j.chom.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalarikkal SP, Sundaram GM. Edible plant-derived exosomal microRNAs: Exploiting a cross-kingdom regulatory mechanism for targeting SARS-CoV-2. Toxicol Appl Pharmacol. 2021;414:115425. doi: 10.1016/j.taap.2021.115425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim VN. MicroRNA biogenesis: coordinated cropping and dicing. Nat Rev Mol Cell Biol. 2005;6:376–385. doi: 10.1038/nrm1644. [DOI] [PubMed] [Google Scholar]
- Kimura K, Hohjoh H, Fukuoka M, et al. Circulating exosomes suppress the induction of regulatory T cells via let-7i in multiple sclerosis. Nat Commun. 2018;9:17. doi: 10.1038/s41467-017-02406-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppers-Lalic D, Hackenberg M, Bijnsdorp IV, et al. Nontemplated nucleotide additions distinguish the small RNA composition in cells from exosomes. Cell Rep. 2014;8:1649–1658. doi: 10.1016/j.celrep.2014.08.027. [DOI] [PubMed] [Google Scholar]
- Kosaka N, Iguchi H, Hagiwara K, et al. Neutral sphingomyelinase 2 (nSMase2)-dependent exosomal transfer of angiogenic microRNAs regulate cancer cell metastasis. J Biol Chem. 2013;288:10849–10859. doi: 10.1074/jbc.M112.446831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka N, Izumi H, Sekine K et al (2010) microRNA as a new immune-regulatory agent in breast milk. Silence 1. 710.1186/1758-907X-1-7 [DOI] [PMC free article] [PubMed]
- Kotlabova K, Doucha J, Hromadnikova I. Placental-specific microRNA in maternal circulation–identification of appropriate pregnancy-associated microRNAs with diagnostic potential. J Reprod Immunol. 2011;89:185–191. doi: 10.1016/j.jri.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Kusuma RJ, Manca S, Friemel T, et al. Human vascular endothelial cells transport foreign exosomes from cow’s milk by endocytosis. Am J Physiol Cell Physiol. 2016;310:C800–807. doi: 10.1152/ajpcell.00169.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasser C, Alikhani VS, Ekstrom K, et al. Human saliva, plasma and breast milk exosomes contain RNA: uptake by macrophages. J Transl Med. 2011;9:9. doi: 10.1186/1479-5876-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leidal AM, Huang HH, Marsh T, et al. The LC3-conjugation machinery specifies the loading of RNA-binding proteins into extracellular vesicles. Nat Cell Biol. 2020;22:187–199. doi: 10.1038/s41556-019-0450-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Li J, Zhang Y, Li D, et al. Small non-coding RNAs transfer through mammalian placenta and directly regulate fetal gene expression. Protein Cell. 2015;6:391–396. doi: 10.1007/s13238-015-0156-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang G, Zhu Y, Sun B, et al. Assessing the survival of exogenous plant microRNA in mice. Food Sci Nutr. 2014;2:380–388. doi: 10.1002/fsn3.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H, Zhang S, Fu Z, et al. Effective detection and quantification of dietetically absorbed plant microRNAs in human plasma. J Nutr Biochem. 2015;26:505–512. doi: 10.1016/j.jnutbio.2014.12.002. [DOI] [PubMed] [Google Scholar]
- Liang X, Zhang L, Wang S, et al. Exosomes secreted by mesenchymal stem cells promote endothelial cell angiogenesis by transferring miR-125a. J Cell Sci. 2016;129:2182–2189. doi: 10.1242/jcs.170373. [DOI] [PubMed] [Google Scholar]
- Lin F, Zeng Z, Song Y, et al. YBX-1 mediated sorting of miR-133 into hypoxia/reoxygenation-induced EPC-derived exosomes to increase fibroblast angiogenesis and MEndoT. Stem Cell Res Ther. 2019;10:263. doi: 10.1186/s13287-019-1377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, da Cunha AP, Rezende RM, et al. The Host Shapes the Gut Microbiota via Fecal MicroRNA. Cell Host Microbe. 2016;19:32–43. doi: 10.1016/j.chom.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Rezende RM, Moreira TG, et al. Oral Administration of miR-30d from Feces of MS Patients Suppresses MS-like Symptoms in Mice by Expanding Akkermansia muciniphila. Cell Host Microbe. 2019;26:779–794e778. doi: 10.1016/j.chom.2019.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, Li H, Li N, et al. MEX3C interacts with adaptor-related protein complex 2 and involves in miR-451a exosomal sorting. PLoS ONE. 2017;12:e0185992. doi: 10.1371/journal.pone.0185992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Wang P, Wang X, et al. Detection of dietetically absorbed maize-derived microRNAs in pigs. Sci Rep. 2017;7:645. doi: 10.1038/s41598-017-00488-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manca S, Upadhyaya B, Mutai E, et al. Milk exosomes are bioavailable and distinct microRNA cargos have unique tissue distribution patterns. Sci Rep. 2018;8:11321. doi: 10.1038/s41598-018-29780-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L, Li X, Gong S, et al. Serum exosomes contain ECRG4 mRNA that suppresses tumor growth via inhibition of genes involved in inflammation, cell proliferation, and angiogenesis. Cancer Gene Ther. 2018;25:248–259. doi: 10.1038/s41417-018-0032-3. [DOI] [PubMed] [Google Scholar]
- Mao S, Sun Q, Xiao H, et al. Secreted miR-34a in astrocytic shedding vesicles enhanced the vulnerability of dopaminergic neurons to neurotoxins by targeting Bcl-2. Protein Cell. 2015;6:529–540. doi: 10.1007/s13238-015-0168-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie AJ, Hoshino D, Hong NH, et al. KRAS-MEK Signaling Controls Ago2 Sorting into Exosomes. Cell Rep. 2016;15:978–987. doi: 10.1016/j.celrep.2016.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moloney GM, Viola MF, Hoban AE, et al. Faecal microRNAs: indicators of imbalance at the host-microbe interface? Benef Microbes. 2018;9:175–183. doi: 10.3920/BM2017.0013. [DOI] [PubMed] [Google Scholar]
- Mu J, Zhuang X, Wang Q, et al. Interspecies communication between plant and mouse gut host cells through edible plant derived exosome-like nanoparticles. Mol Nutr Food Res. 2014;58:1561–1573. doi: 10.1002/mnfr.201300729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orom UA, Nielsen FC, Lund AH. MicroRNA-10a binds the 5’UTR of ribosomal protein mRNAs and enhances their translation. Mol Cell. 2008;30:460–471. doi: 10.1016/j.molcel.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Pan C, Zhu D, Wang Y, et al. Human Cytomegalovirus miR-UL148D Facilitates Latent Viral Infection by Targeting Host Cell Immediate Early Response Gene 5. PLoS Pathog. 2016;12:e1006007. doi: 10.1371/journal.ppat.1006007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park NJ, Zhou H, Elashoff D, et al. Salivary microRNA: discovery, characterization, and clinical utility for oral cancer detection. Clin Cancer Res. 2009;15:5473–5477. doi: 10.1158/1078-0432.CCR-09-0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck BC, Mah AT, Pitman WA, et al. Functional Transcriptomics in Diverse Intestinal Epithelial Cell Types Reveals Robust MicroRNA Sensitivity in Intestinal Stem Cells to Microbial Status. J Biol Chem. 2017;292:2586–2600. doi: 10.1074/jbc.M116.770099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, et al. Functional delivery of viral miRNAs via exosomes. Proc Natl Acad Sci U S A. 2010;107:6328–6333. doi: 10.1073/pnas.0914843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Boza J, Boeckx A, Lion M, et al. hnRNPA2B1 inhibits the exosomal export of miR-503 in endothelial cells. Cell Mol Life Sci. 2020;77:4413–4428. doi: 10.1007/s00018-019-03425-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruegger S, Grosshans H. MicroRNA turnover: when, how, and why. Trends Biochem Sci. 2012;37:436–446. doi: 10.1016/j.tibs.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Sabit H, Cevik E, Tombuloglu H, et al. Triple negative breast cancer in the era of miRNA. Crit Rev Oncol Hematol. 2021;157:103196. doi: 10.1016/j.critrevonc.2020.103196. [DOI] [PubMed] [Google Scholar]
- Santangelo L, Giurato G, Cicchini C, et al. The RNA-Binding Protein SYNCRIP Is a Component of the Hepatocyte Exosomal Machinery Controlling MicroRNA Sorting. Cell Rep. 2016;17:799–808. doi: 10.1016/j.celrep.2016.09.031. [DOI] [PubMed] [Google Scholar]
- Selth LA, Townley S, Gillis JL (2012) Discovery of circulating microRNAs associated with huaman prostate cancer using amouse model of disease. Int J Cancer 131:652–661 [DOI] [PubMed]
- Shen CB, Yu L, Gu YN, et al. Inhibited expression of GATA-3 on Th2 cells transfect Astragalus-derived miR-396 of asthmatic mice in vivo. Chin J Immunol. 2019;35:3001–3007. doi: 10.3969/j.issn.1000-484X.2019.24.011. [DOI] [Google Scholar]
- Shen S, Song Y, Zhao B, et al. Cancer-derived exosomal miR-7641 promotes breast cancer progression and metastasis. Cell Commun Signal. 2021;19:20. doi: 10.1186/s12964-020-00700-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shurtleff MJ, Temoche-Diaz MM, Karfilis KV et al (2016) Y-box protein 1 is required to sort microRNAs into exosomes in cells and in a cell-free reaction. Elife 5. 10.7554/eLife.19276 [DOI] [PMC free article] [PubMed]
- Squadrito ML, Baer C, Burdet F, et al. Endogenous RNAs modulate microRNA sorting to exosomes and transfer to acceptor cells. Cell Rep. 2014;8:1432–1446. doi: 10.1016/j.celrep.2014.07.035. [DOI] [PubMed] [Google Scholar]
- Sun Z, Shi K, Yang S, et al. Effect of exosomal miRNA on cancer biology and clinical applications. Mol Cancer. 2018;17:147. doi: 10.1186/s12943-018-0897-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabet F, Vickers KC, Cuesta Torres LF, et al. HDL-transferred microRNA-223 regulates ICAM-1 expression in endothelial cells. Nat Commun. 2014;5:3292. doi: 10.1038/ncomms4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi RU, Prieto-Vila M, Kohama I, et al. Development of miRNA-based therapeutic approaches for cancer patients. Cancer Sci. 2019;110:1140–1147. doi: 10.1111/cas.13965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarallo S, Ferrero G, De Filippis F, et al. Stool microRNA profiles reflect different dietary and gut microbiome patterns in healthy individuals. Gut. 2021 doi: 10.1136/gutjnl-2021-325168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temoche-Diaz MM, Shurtleff MJ, Nottingham RM et al (2019) Distinct mechanisms of microRNA sorting into cancer cell-derived extracellular vesicle subtypes. Elife 8. 10.7554/eLife.47544 [DOI] [PMC free article] [PubMed]
- Teng Y, Ren Y, Hu X, et al. MVP-mediated exosomal sorting of miR-193a promotes colon cancer progression. Nat Commun. 2017;8:14448. doi: 10.1038/ncomms14448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng Y, Ren Y, Sayed M, et al. Plant-Derived Exosomal MicroRNAs Shape the Gut Microbiota. Cell Host Microbe. 2018;24:637–652e638. doi: 10.1016/j.chom.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng Y, Xu F, Zhang X, et al. Plant-derived exosomal microRNAs inhibit lung inflammation induced by exosomes SARS-CoV-2 Nsp12. Mol Ther. 2021;29:2424–2440. doi: 10.1016/j.ymthe.2021.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y, Cai L, Tian Y, et al. miR156a Mimic Represses the Epithelial-Mesenchymal Transition of Human Nasopharyngeal Cancer Cells by Targeting Junctional Adhesion Molecule A. PLoS ONE. 2016;11:e0157686. doi: 10.1371/journal.pone.0157686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers KC, Palmisano BT, Shoucri BM, et al. MicroRNAs are Transported in Plasma and Delivered to Recipient Cells by High-Density Lipoproteins. Nat Cell Biol. 2011;13:423–433. doi: 10.1038/ncb2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarroya-Beltri C, Gutierrez-Vazquez C, Sanchez-Cabo F, et al. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat Commun. 2013;4:2980. doi: 10.1038/ncomms3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtue AT, McCright SJ, Wright JM et al (2019) The gut microbiota regulates white adipose tissue inflammation and obesity via a family of microRNAs. Sci Transl Med 11. 10.1126/scitranslmed.aav1892 [DOI] [PMC free article] [PubMed]
- Wagner J, Riwanto M, Besler C, et al. Characterization of levels and cellular transfer of circulating lipoprotein-bound microRNAs. Arterioscler Thromb Vasc Biol. 2013;33:1392–1400. doi: 10.1161/ATVBAHA.112.300741. [DOI] [PubMed] [Google Scholar]
- Wu B, Su S, Patil DP, et al. Molecular basis for the specific and multivariant recognitions of RNA substrates by human hnRNP A2/B1. Nat Commun. 2018;9:420. doi: 10.1038/s41467-017-02770-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Fan J, Belasco JG. MicroRNAs direct rapid deadenylation of mRNA. Proc Natl Acad Sci U S A. 2006;103:4034–4039. doi: 10.1073/pnas.0510928103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu TL, Sun YW, Feng XY, et al. Development of miRNA-Based Approaches to Explore the Interruption of Mosquito-Borne Disease Transmission. Front Cell Infect Microbiol. 2021;11:665444. doi: 10.3389/fcimb.2021.665444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Farmer LM, Agyekum AA, et al. Detection of an Abundant Plant-Based Small RNA in Healthy Consumers. PLoS ONE. 2015;10:e0137516. doi: 10.1371/journal.pone.0137516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Farmer LM, Agyekum AA, et al. Detection of dietary plant-based small RNAs in animals. Cell Res. 2015;25:517–520. doi: 10.1038/cr.2015.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yekta S, Shih IH, Bartel DP. MicroRNA-directed cleavage of HOXB8 mRNA. Science. 2004;304:594–596. doi: 10.1126/science.1097434. [DOI] [PubMed] [Google Scholar]
- Yin Y, Cai X, Chen X, et al. Tumor-secreted miR-214 induces regulatory T cells: a major link between immune evasion and tumor growth. Cell Res. 2014;24:1164–1180. doi: 10.1038/cr.2014.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B, Yang Z, Li J, et al. Methylation as a crucial step in plant microRNA biogenesis. Science. 2005;307:932–935. doi: 10.1126/science.1107130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu WY, Cai W, Ying HZ, et al. Exogenous Plant gma-miR-159a, Identified by miRNA Library Functional Screening, Ameliorated Hepatic Stellate Cell Activation and Inflammation via Inhibiting GSK-3beta-Mediated Pathways. J Inflamm Res. 2021;14:2157–2172. doi: 10.2147/JIR.S304828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zernecke A, Bidzhekov K, Noels H, et al. Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Sci Signal. 2009;2:ra81. doi: 10.1126/scisignal.2000610. [DOI] [PubMed] [Google Scholar]
- Zhang L, Hou D, Chen X, et al. Exogenous plant MIR168a specifically targets mammalian LDLRAP1: evidence of cross-kingdom regulation by microRNA. Cell Res. 2012;22:107–126. doi: 10.1038/cr.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Zhang S, Yao J, et al. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature. 2015;527:100–104. doi: 10.1038/nature15376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liu D, Chen X, et al. Secreted monocytic miR-150 enhances targeted endothelial cell migration. Mol Cell. 2010;39:133–144. doi: 10.1016/j.molcel.2010.06.010. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Xing T, Chen Y, et al. Exosome-mediated miR-200b promotes colorectal cancer proliferation upon TGF-β1 exposure. Biomed Pharmacother. 2018;106:1135–1143. doi: 10.1016/j.biopha.2018.07.042. [DOI] [PubMed] [Google Scholar]
- Zhou LK, Zhou Z, Jiang XM, et al. Absorbed plant MIR2911 in honeysuckle decoction inhibits SARS-CoV-2 replication and accelerates the negative conversion of infected patients. Cell Discov. 2020;6:54. doi: 10.1038/s41421-020-00197-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Ren H, Dai B, et al. Hepatocellular carcinoma-derived exosomal miRNA-21 contributes to tumor progression by converting hepatocyte stellate cells to cancer-associated fibroblasts. J Exp Clin Cancer Res. 2018;37:324. doi: 10.1186/s13046-018-0965-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Li X, Liu J, et al. Honeysuckle-encoded atypical microRNA2911 directly targets influenza A viruses. Cell Res. 2015;25:39–49. doi: 10.1038/cr.2014.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu K, Liu M, Fu Z, et al. Plant microRNAs in larval food regulate honeybee caste development. PLoS Genet. 2017;13:e1006946. doi: 10.1371/journal.pgen.1006946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Yu S, Qiu HJ, et al. [Exosomes: another arena for the game between viruses and hosts] Sheng Wu Gong Cheng Xue Bao. 2020;36:1732–1740. doi: 10.13345/j.cjb.200039. [DOI] [PubMed] [Google Scholar]
- Zhu Z, Huang J, Li X, et al. Gut microbiota regulate tumor metastasis via circRNA/miRNA networks. Gut Microbes. 2020;12:1788891. doi: 10.1080/19490976.2020.1788891. [DOI] [PMC free article] [PubMed] [Google Scholar]


