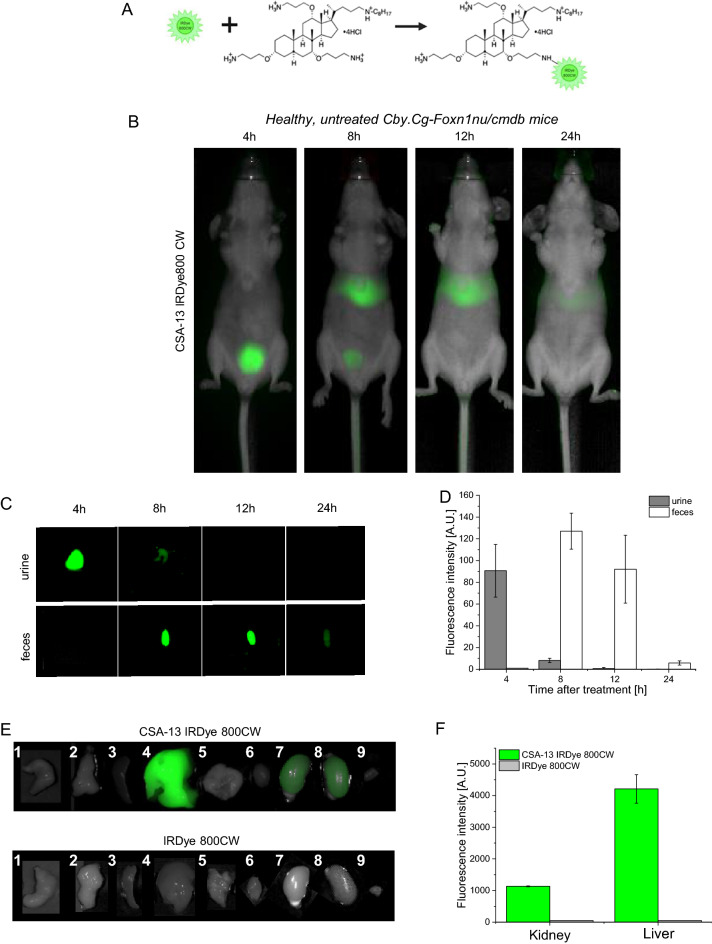
Figure 3.
Scheme description of CSA-13 labeling with IRDye 800CW (A). Biodistribution of intravenously administrated CSA-13 labeled with IRDye®800CW (CSA-13-IRDye800CW) estimated by fluorescence-based analysis of CSA-13-IRDye800CW-targeted fluorescence signal in healthy, non-infected Cby.Cg-Foxn1nu/cmdb mice (n = 5; group 8) 4, 8, 12 and 24 h post injection of CSA-13 IRDye800CW. Results from representative animals are shown (B). Representative scans of urine and feces collected from healthy mice (n = 5; group 8) 4, 8, 12 and 24 h after injection of CSA-13-IRDye800CW (C). Presence of labeled CSA-13 in urine and feces was estimated based on fluorescence intensity of collected excreta and presented as mean value ± SEM from all areas of each urine and feces (D). Organ uptake of CSA-13-IRDye800CW (group 8) and IRDye800CW (group 9) after 24 h post its administration was estimated based on fluorescence intensity of collected organs (1—stomach, 2—pancreas, 3—spleen, 4—liver, 5—lungs, 6—heart, 7—left kidney, 8—right kidney, 9—bladder) and presented as mean value ± SEM from all areas of each organs (E, F).