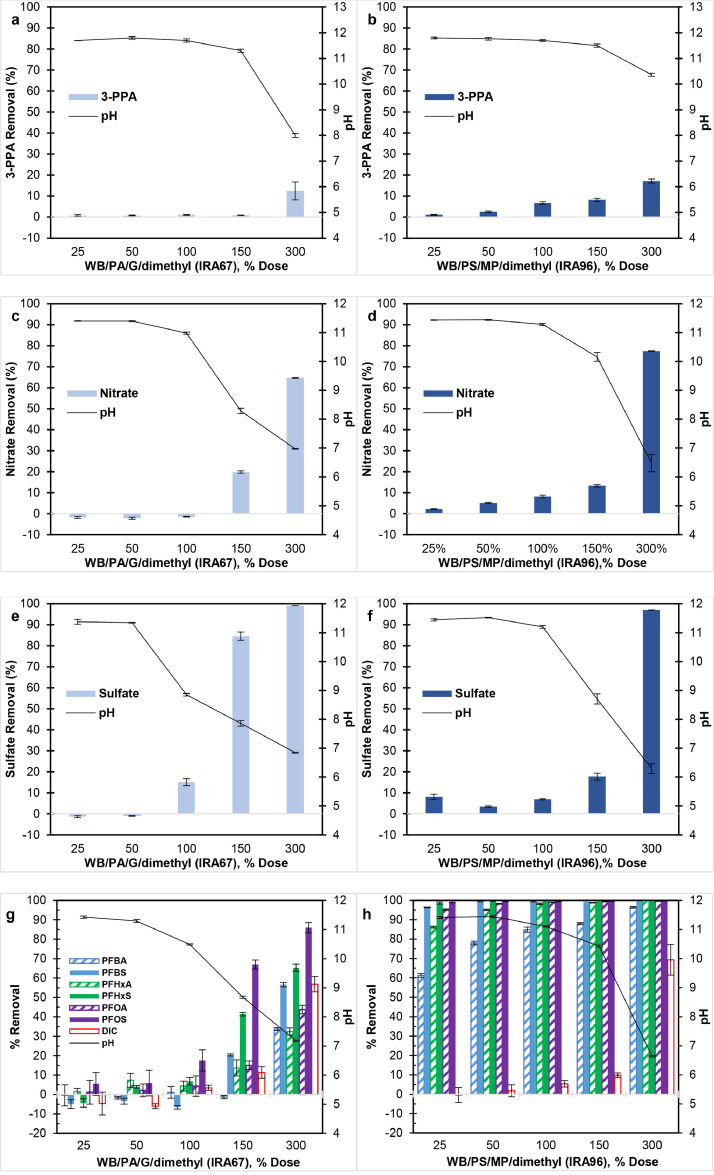
Fig. 1.
Impact of solution pH on contaminant removal by weak-base anion exchange resins for (a, b) 3-phenylpropionic acid, (c, d) nitrate, and (e, f) sulfate in single-solute system (C0 ≈ 2.14 meq/L) and (g, h) the six PFAAs in the presence of sodium bicarbonate (C0 ≈ 2.14 meq/L) in multi-solute system. Initial concentration of each PFAA was C0 = 80 μg/L ( = 480 μg/L). Resins were first equilibrated for 24 h under basic conditions (pH ≈ 11) then placed in test water at: (a, b) pH 11.7, (c, d) pH 11.4, (e, f) pH 11.4, and (g,h) pH 11.4. Error bars show one standard deviation.