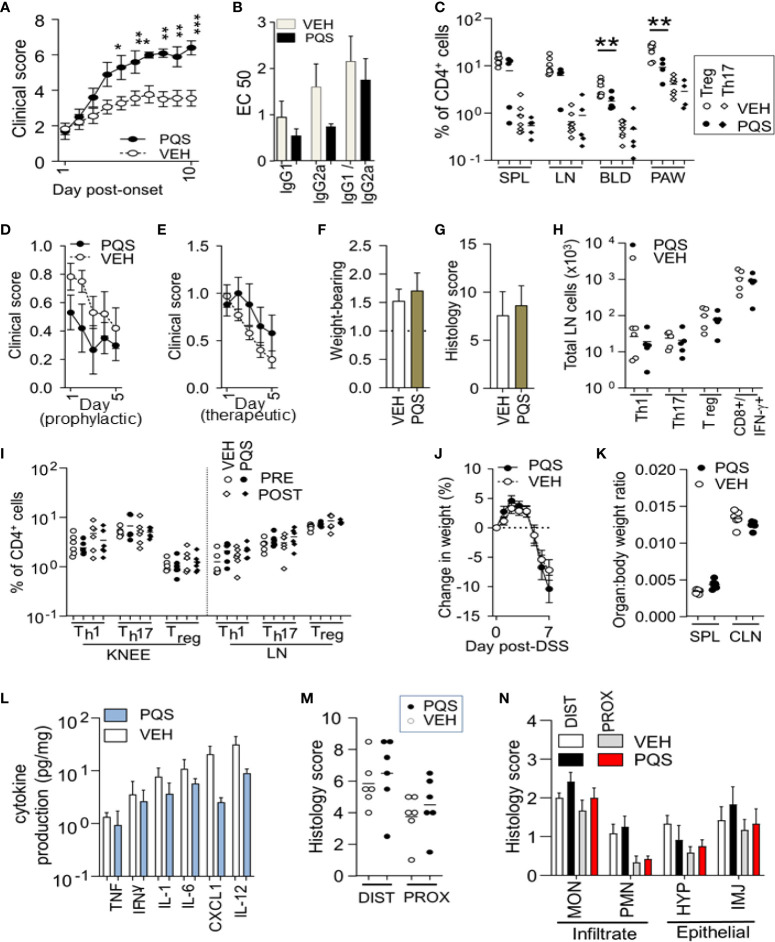
Figure 1.
The effect of PQS differs between in vivo models of disease. PQS modulation of disease activity in CIA (A–C), AIA (D–I) and DSS (J–N). (A): Clinical severity from the onset of paw swelling (day 1) to the termination of the experiment (day 10); (B) Anti-collagen humoral responses for IgG1 and IgG2a antibodies and their ratio IgG2a:IgG1; titres were measured by a dilution assay and the results expressed as EC50; *p<0.05, **p<0.01, ***p<0.001 (n = 8 vehicle, n = 5 PQS); (C) Analysis of Th17+ or CD4+FoxP3+ Treg cells in affected paws (PAW), lymph nodes (LN) spleens (SPL) and blood (BLD); **P<0.01 (vehicle n = 8; PQS n = 5); (D) score (change in knee width) after immunisation and (E) after intra-articular injection; n = 5; (F) Weight-bearing and (G) histological scores of joint damage in AIA mice; (H) Th1, Th17 or Treg cells in the inguinal lymph nodes of vehicle or PQS-treated animals; n = 6; (I) CD4+ Th1, Th17 and FoxP3+ T cells in the arthritic knee or lymph nodes of mice with AIA; n = 6; (J) Dextran sulphate induced changes in body weight, n = 6; (K) Ratio of the weights of spleen or colon with total body weight; n = 6; (L) Log cytokine production by ex vivo colon cultures for TNF, IFN-γ, IL-1β, IL-6, CXCL1 and IL-12 (n = 6); (M) Overall histological assessment of the distal (DIST) and proximal (PROX) colon; (N) Analysis of infiltrating monocytes (MON) or granulocytes (PMN), and the hyperplasia (HYP) or injury (INJ) of intestinal epithelial cells (n = 6).