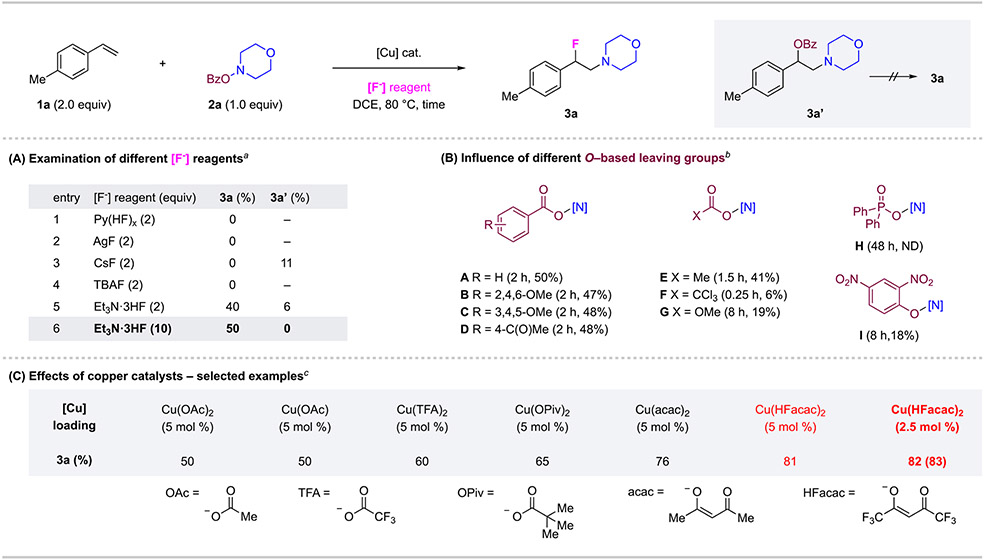
Figure 2. Condition Development for Alkene Aminofluorination Reaction.
Conditions: 1a (2.0 equiv), 2a (0.2 mmol, 1.0 equiv), [F−] reagent, DCE (1.0 mL), 80 °C, 2 h, in 10-mL sealed FEP tube. Yields were determined by 1H NMR of the crude mixture with dibromomethane as an internal standard. Isolated yields shown in parentheses. aCu(OAc)2 (10 mol %) used. bCu(OAc)2 (10 mol %) Et3N·3HF (10 equiv), hydroxylamine derivative (1.0 equiv). c2a (1.0 equiv), Et3N·3HF (10 equiv).